oLAB (Anti-Oxidized LDL Autoantibodies) ELISA | BI-20032
-
Method
Indirect ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
Serum
-
Sample volume
50 µl / well
-
Assay time
1.5 h / 30 min / 15 min
-
Sensitivity
48 mU/ml
-
Standard range
0 – 1,200 mU/ml
-
Specificity
Anti-oxidized LDL autoantibodies
-
Precision
In-between-run (n=5): ≤ 8 % CV
Within-run (n=8): ≤ 4 % CV
-
Use
Research use only
Anti-Oxidized LDL Autoantibodies Product Overview
The anti-oxidized LDL autoantibodies (oLAB) immunoassay is a 2 hour 15 minutes, 96-well indirect ELISA for the quantitative determination of anti-oxidized LDL autoantibodies in serum.
Anti-Oxidized LDL Autoantibodies Principle of the Assay
The anti-oxldl antibody ELISA kit is an indirect enzyme immunoassay for the quantitative determination of anti-oxidized LDL autoantibodies in serum samples.
In a first step, prediluted standard/control/sample are pipetted into the wells of the microtiter strips, which are pre-coated with oxidized LDL antigen. Anti-oxidized LDL autoantibodies present in the standard/control/sample bind to the pre-coated antigen in the well. After a washing step, which removes all non-specific unbound material, the conjugate (monoclonal anti-human IgG-HRP) is pipetted into the wells and reacts with the anti-oxidized LDL autoantibodies.
After another washing step, the substrate (TMB, tetramethylbenzidine) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is directly proportional to the amount of anti-oxidized LDL autoantibodies present in the sample. This color change is detectable with a standard microplate reader. The concentration of anti-oxidized LDL autoantibodies in the sample is determined directly from the dose response curve.
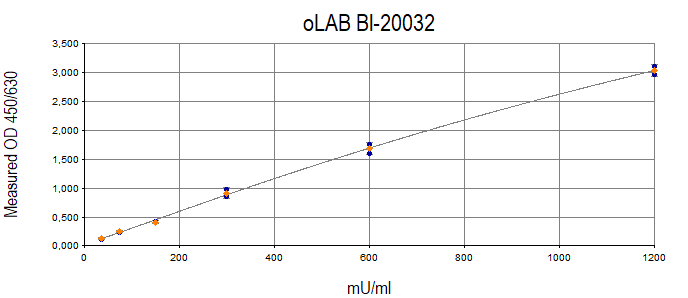
Anti-Oxidized LDL Autoantibodies Typical Standard Curve
The figure below shows a typical standard curve for oxldl assay kit.
Anti-Oxidized LDL Autoantibodies ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Oxidized LDL precoated microtiter strips in strip holder, packed in aluminum bag with desiccant |
12 x 8 tests |
|
DILPLATE |
Uncoated microtiter plate for sample pre-treatment |
12 x 8 wells |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STD |
Standards 1-6 ( 0; 0.62; 1.25; 2.5; 5; 10 μg/ml), anti-oxidized LDL IgG antibodies, white caps, ready to use |
6 x 500 µl |
|
CTRL |
Control A and B, yellow caps, ready to use, exact concentrations see labels |
2 x 500 µl |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 60 ml |
|
CONJ |
Conjugate, (monoclonal anti-human IgG-HRP), amber cap, ready to use |
1 x 13 ml |
|
SUB |
Substrate (TMB solution), blue cap, ready to use |
1 x 13 ml |
|
STOP |
Stop solution, white cap, ready to use |
1 x 7 ml |
Serum is suitable for use in this oxldl assay kit. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Serum
Collect venous blood samples in standardized serum separator tubes (SST). Allow samples to clot for 30 minutes at room temperature. Perform separation by centrifugation according to the tube manufacturer’s instructions for use. Assay the acquired samples immediately or aliquot and store at -25°C or lower. Lipemic or haemolyzed samples may give erroneous results. Do not freeze-thaw samples more than four times.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature (18-26°C). |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
Anti-Oxidized LDL Autoantibodies Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring samples and reagents to room temperature (18-24°C). |
|
2. |
Mark positions for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
In the Predilution Plate
Note: All STD /CTRL/SAMPLE (standard/control/sample) must be used in 1:55 end-dilution in the assay (pre-dilution 1:5 + assay-dilution 1:11). Use the enclosed DILPLATE (uncoated microtiter plate) for the 1:5 predilution step.
|
1. |
Pipette 200 µl ASYBUF (assay buffer) into the appropriate wells of the uncoated microtiter plate. . |
|
2. |
Add 50 µl STD/CTRL/SAMPLE (standard/control/sample) into the respective wells, mix well (= 1:5 dilution). |
In the Pre-Coated Plate
|
1. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
2. |
Pipette 200 µl ASYBUF (assay buffer, red cap) into each well, including blank. |
|
3. |
Add 20 µl 1:5 prediluted STD/CTRL/SAMPLE into the respective wells. Swirl gently. Note: The transfer of the prediluted STD /CTRL/SAMPLE into the precoated microtiter strips must be completed within 15 minutes. Use a multichannel pipette. |
|
4. |
Cover the plate tightly and incubate for 1.5 hours at 37°C. |
|
5. |
Aspirate and wash wells 4 x with 300 µl diluted WASHBUF (wash buffer). After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
6. |
Add 100 µl CONJ (conjugate, amber cap) into each well except blank. |
|
7. |
Cover tightly and incubate for 30 minutes at room temperature (18-24°C). |
|
8. |
Aspirate and wash wells 4 x with 300 µl diluted WASHBUF. After the final wash, remove remaining WASHBUF by strongly tapping plate against a paper towel. |
|
9. |
Add 100 µl SUB (substrate, blue cap) into each well. |
|
10. |
Incubate for 15 min at room temperature in the dark. |
|
11. |
Add 50 µl STOP (stop solution, white cap) into each well. Swirl gently. |
| 12. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Subtract the absorbance read-out obtained for the blank from all other values. Construct a standard curve from the values of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and will need to be evaluated by the user.
Obtain sample concentrations from the standard curve. Samples for which the optical density (OD) value exceeds the highest point of the standard range can be diluted further. Sample predilutions other than 1:5 have to be considered when calculating the final concentration of the sample.
The quality control protocol supplied with the kit shows the results of the final release QC for each kit. Data for optical density obtained by customers may differ due to various influences including the normal decrease of signal intensity throughout shelf life. However, this does not affect validity of results as long as an optical density of 1.00 or higher is obtained for the standard with the highest concentration and the values of the CTRLs are within the target range (see labels).
Anti-Oxidized LDL Autoantibodies
Oxidized low density lipoprotein (oxLDL) is believed to play a critical role in the development and progression of atherosclerosis. Accumulation of oxLDL in macrophages and smooth muscle cells causes foam cell formation, an initial step in the disease. Autoantibodies against oxidatively modified LDL can be used as a parameter that consistently mirrors the occurrence of oxidation processes taking place in vivo. In fact, elevated levels of autoantibodies against oxLDL have been detected in the blood stream of patients with coronary artery disease. Moreover, recent studies indicate a correlation between autoantibodies against oxLDL and the progression of carotid atherosclerosis. Increased serum concentrations of oLAB have also been described in various diseases such as pre-eclampsia and systemic lupus erythematosus. Decreased oLAB titers were observed during septicemia and myocardial infarction.
An overview on the clinical applications of oLAB has been published.
-
Cardiovascular Disease
- Artherosclerosis (Bornaun et al., 2017; de Freitas et al., 2018; Pawlak et al., 2012; Shoji et al., 2003; Stanek et al., 2018)
- Coronary artery disease (Faviou et al., 2005; Miller et al., 2003)
- Myocardial infarction (Huang et al., 2013)
- Stroke (Ciancarelli et al., 2012)
-
Other
- Acute septicaemia (Al-Banna and Lehmann, 2013)
- Preeclampsia (Arifin et al., 2017)
Literature
Is oxidized low-density lipoprotein the connection between atherosclerosis, cardiovascular risk and nephrolithiasis?
de Freitas, A.C.P., Torres, L.C., Duarte, M. do C.M.B., da Matta, M.C., Casarini, D.E., Schor, N., 2018. Urolithiasis.
Whole-Body Cryotherapy Decreases the Levels of Inflammatory, Oxidative Stress, and Atherosclerosis Plaque Markers in Male Patients with Active-Phase Ankylosing Spondylitis in the Absence of Classical Cardiovascular Risk Factors.
Stanek, A., Cholewka, A., Wielkoszyński, T., Romuk, E., Sieroń, A., 2018. Mediators Inflamm. 2018, 1–11.
Increased circulating oxidised low-density lipoprotein and antibodies to oxidised low-density lipoprotein in preeclampsia.
Arifin, R., Kyi, W.M., Che Yaakob, C.A., Yaacob, N.M., 2017. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 37, 580–584.
Assessment of lipid profile and some risk factors of atherosclerosis in children whose parents had early onset coronary artery disease.
Bornaun, H., Öner, N., Nişli, K., Öztarhan, K., Yavuz, T., Türkoğlu, Ü., Dindar, A., Eker Ömeroglu, R., 2017. Arch. Argent. Pediatr. 115, 50–54.
Oxidized LDL and LOX-1 in Experimental Sepsis.
Al-Banna, N., Lehmann, C., 2013. Mediators Inflamm.
Using Oxidized Low-Density Lipoprotein Autoantibodies to Predict Restenosis after Balloon Angioplasty in Patients with Acute Myocardial Infarction.
Huang, C.-H., Chang, C.-C., Huang, C.-S., Kuo, C.-L., Chen, C.-P., Hsia, C.-H., Chang, Y.-M., Chen, H.-T., Feng, C.-C., Lin, L.-S., Yang, P.-T., Tsai, C.-D., Lin, C.-S., Liu, C.-S., 2013. PLoS ONE 8, e74726.
Oxidative stress in post-acute ischemic stroke patients after intensive neurorehabilitation.
Ciancarelli, I., De Amicis, D., Di Massimo, C., Carolei, A., Ciancarelli, M.G.T., 2012. Curr. Neurovasc. Res. 9, 266–273
Oxidized LDL to autoantibodies against oxLDL ratio – The new biomarker associated with carotid atherosclerosis and cardiovascular complications in dialyzed patients.
Pawlak, K., Mysliwiec, M., Pawlak, D., 2012. Atherosclerosis 224, 252–257.
Circulating oxidized low density lipoprotein, autoantibodies against them and homocysteine serum levels in diagnosis and estimation of severity of coronary artery disease.
Faviou, E., Vourli, G., Nounopoulos, C., Zachari, A., Dionyssiou-Asteriou, A., 2005. Free Radic. Res. 39, 419–429
Antibodies to oxidized low-density lipoprotein in patients following coronary artery revascularization.
Miller, E.R., Erlinger, T.P., Blumenthal, R.S., Margolis, S., Allen, J.K., 2003. Coron. Artery Dis. 14, 163–169.
The association of antibodies against oxidized low-density lipoprotein with atherosclerosis in hemodialysis patients.
Shoji, T., Kimoto, E., Shinohara, K., Emoto, M., Ishimura, E., Miki, T., Tsujimoto, Y., Tabata, T., Nishizawa, Y., 2003. Kidney Int. Suppl. S128-130.
-
Is oxidized low-density lipoprotein the connection between atherosclerosis, cardiovascular risk and nephrolithiasis? de Freitas, A.C.P., Torres, L.C., Duarte, M. do C.M.B., da Matta, M.C., Casarini, D.E., Schor, N., 2019. Urolithiasis 47, 347–356.
- The impact of antihypertensive pharmacotherapy on interplay between protein-bound uremic toxin (indoxyl sulfate) and markers of inflammation in patients with chronic kidney disease.
Kaminski, T.W., Pawlak, K., Karbowska, M., Znorko, B., Mor, A.L., Mysliwiec, M., Pawlak, D., 2019. Int Urol Nephrol.
- Novel approaches for the assessment of relative body weight and body fat in diagnosis and treatment of anorexia nervosa: A cross-sectional study.
Lackner, S., Mörkl, S., Müller, W., Fürhapter-Rieger, A., Oberascher, A., Lehofer, M., Bieberger, C., Wonisch, W., Amouzadeh-Ghadikolai, O., Moser, M., Mangge, H., Zelzer, S., Holasek, S.J., 2019. Clinical Nutrition.
- Upregulation of the Nitrosylome in Bipolar Disorder Type 1 (BP1), but not BP2, and Major Depression: Increased IgM Antibodies to Nitrosylated Conjugates are Associated with Indicants of Leaky Gut.
Maes, M., Simeonova, D., Stoyanov, D., Leunis, J.-C., 2019.
- Is oxidized low-density lipoprotein the connection between atherosclerosis, cardiovascular risk and nephrolithiasis?
de Freitas, A.C.P., Torres, L.C., Duarte, M. do C.M.B., da Matta, M.C., Casarini, D.E., Schor, N., 2018. Urolithiasis.
PMID:30302491
- Metabolic disorders and inflammation are associated with familial combined hyperlipemia.
Díaz-Ruiz, M., Martínez-Triguero, M.L., López-Ruiz, A., la Cruz, F.F., Bañuls, C., Hernández-Mijares, A., 2018. Clin. Chim. Acta.
PMID:30201373
- Bipolar disorder in youth is associated with increased levels of vitamin D-binding protein.
Petrov, B., Aldoori, A., James, C., Yang, K., Algorta, G.P., Lee, A., Zhang, L., Lin, T., Awadhi, R.A., Parquette, J.R., Samogyi, A., Arnold, L.E., Fristad, M.A., Gracious, B., Ziouzenkova, O., 2018. Transl Psychiatry 8, 61.
PMID:29531242 PMCID: PMC5847532
- Whole-Body Cryotherapy Decreases the Levels of Inflammatory, Oxidative Stress, and Atherosclerosis Plaque Markers in Male Patients with Active-Phase Ankylosing Spondylitis in the Absence of Classical Cardiovascular Risk Factors.
Stanek, A., Cholewka, A., Wielkoszyński, T., Romuk, E., Sieroń, A., 2018. Mediators of Inflammation 2018, 1–11.
- Increased circulating oxidised low-density lipoprotein and antibodies to oxidised low-density lipoprotein in preeclampsia.
Arifin, R., Kyi, W.M., Che Yaakob, C.A., Yaacob, N.M., 2017. J Obstet Gynaecol 37, 580–584.
PMID:28358592
- Assessment of lipid profile and some risk factors of atherosclerosis in children whose parents had early onset coronary artery disease.
Bornaun, H., Öner, N., Nişli, K., Öztarhan, K., Yavuz, T., Türkoğlu, Ü., Dindar, A., Eker Ömeroglu, R., 2017a. Arch Argent Pediatr 115, 50–54.
PMID:28097840
- Evaluación del lipidograma y ciertos factores de riesgo de ateroesclerosis en niños cuyos padres tuvieron arteriopatía coronaria de inicio temprano.
Bornaun, H., Öner, N., Nişli, K., Öztarhan, K., Yavuz, T., Türkoğlu, Ü., Dindar, A., Eker Ömeroglu, R., 2017b. Archivos argentinos de pediatría 115, 50–57.
- Assessment of oxLDL, anti-oxLDL antibodies and lipoprotein-associated phospholipase A2 as cardiovascular risk markers in obese adolescents with and without T1DM.
Omar, N.N., EL Hefnawy, M.H., EL Soda, M.F., Heider, N.M., Hamed, H.I., 2017. Bulletin of Faculty of Pharmacy, Cairo University 55, 325–331.
- Cultivation and Immortalization of Human B-Cells Producing a Human Monoclonal IgM Antibody Binding to MDA-LDL: Further Evidence for Formation of Atherogenic MDA-LDL Adducts in Humans In Vivo.
Tatzber, F., Pursch, E., Resch, U., Pfragner, R., Holasek, S., Lindschinger, M., Cvirn, G., Wonisch, W., 2017. Oxidative Medicine and Cellular Longevity 2017, 1–7.
- Effects of pomegranate juice consumption on oxidative stress in patients with type 2 diabetes: a single-blind, randomized clinical trial.
Sohrab, G., Ebrahimof, S., Sotoudeh, G., Neyestani, T.R., Angoorani, P., Hedayati, M., Siasi, F., 2017. Int J Food Sci Nutr 68, 249–255.
PMID:27633135
- DNA methylation patterns associated with oxidative stress in an ageing population. Hedman, Å.K., Zilmer, M., Sundström, J., Lind, L., Ingelsson, E., 2016. BMC Medical Genomics 9, 72.
- Paraoxonase 1 activity and level of antibodies directed against oxidized low density lipoproteins in a group of an elderly population in Poland – PolSenior study.
Bednarska-Makaruk, M., Rodo, M., Szirkowiec, W., Mossakowska, M., Puzianowska-Kuźnicka, M., Skalska, A., Zdrojewski, T., Ryglewicz, D., Wehr, H., 2015. Archives of Gerontology and Geriatrics 60, 153–161.
- High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis.
Bourji, K., Meyer, A., Chatelus, E., Pincemail, J., Pigatto, E., Defraigne, J.-O., Singh, F., Charlier, C., Geny, B., Gottenberg, J.-E., Punzi, L., Cozzi, F., Sibilia, J., 2015. Free Radic. Biol. Med. 87, 282–289.
PMID:26143738
- Metabolic and Inflammatory Profiles of Biomarkers in Obesity, Metabolic Syndrome, and Diabetes in a Mediterranean Population. DARIOS Inflammatory Study.
Fernández-Bergés, D., Consuegra-Sánchez, L., Peñafiel, J., Cabrera de León, A., Vila, J., Félix-Redondo, F.J., Segura-Fragoso, A., Lapetra, J., Guembe, M.J., Vega, T., Fitó, M., Elosua, R., Díaz, O., Marrugat, J., 2014. Revista Española de Cardiología (English Edition) 67, 624–631.
- Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population.
Gómez, M., Vila, J., Elosua, R., Molina, L., Bruguera, J., Sala, J., Masià, R., Covas, M.I., Marrugat, J., Fitó, M., 2014. Atherosclerosis 232, 134–140.
PMID:24401227
- Oxidized low-density lipoprotein antibodies in myocardial infarction patients without classical risk factors.
Gómez, M., Molina, L., Bruguera, J., Sala, J., Masià, R., Muñoz-Aguayo, D., Tomás, M., Heredia, S., Blanchart, G., Gaixas, S., Vila, J., Fitó, M., 2014. J Cardiovasc Med (Hagerstown) 15, 417–422.
PMID:23877206
- Effect of lifestyle changes and atorvastatin administration on dyslipidemia in hemodialysis patients: a prospective study.
Grzegorzewska, A.E., Niepolski, L., Sikora, J., Janków, M., Jagodziński, P.P., Sowińska, A., 2014. Polish Archives of Internal Medicine 124, 443–451.
- Analysis of 27 vascular-related proteins reveals that NT-proBNP is a potential biomarker for Alzheimer’s disease and mild cognitive impairment: A pilot-study.
Marksteiner, J., Imarhiagbe, D., Defrancesco, M., Deisenhammer, E.A., Kemmler, G., Humpel, C., 2014. Experimental Gerontology 50, 114–121.
- Anti-oxLDL antibodies are clinically insignificant for stroke patients.
Masztalewicz, M., Nowacki, P., Kotlęga, D., Bajer-Czajkowska, A., Drechsler, H., 2014. Neurol. Res. 36, 86–91.
PMID:24107551
- Antioxidant supplementation attenuates oxidative stress in patients undergoing coronary artery bypass graft surgery.
Stanger, O., Aigner, I., Schimetta, W., Wonisch, W., 2014. Tohoku J. Exp. Med. 232, 145–154.
PMID:24573122
- Using Oxidized Low-Density Lipoprotein Autoantibodies to Predict Restenosis after Balloon Angioplasty in Patients with Acute Myocardial Infarction.
Huang, C.-H., Chang, C.-C., Huang, C.-S., Kuo, C.-L., Chen, C.-P., Hsia, C.-H., Chang, Y.-M., Chen, H.-T., Feng, C.-C., Lin, L.-S., Yang, P.-T., Tsai, C.-D., Lin, C.-S., Liu, C.-S., 2013. PLoS ONE 8, e74726.
- In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes.
Maes, M., Kubera, M., Leunis, J.-C., Berk, M., Geffard, M., Bosmans, E., 2013. Acta Psychiatr Scand 127, 344–354.
PMID:22900942
- Oxidative stress in post-acute ischemic stroke patients after intensive neurorehabilitation.
Ciancarelli, I., De Amicis, D., Di Massimo, C., Carolei, A., Ciancarelli, M.G.T., 2012. Curr Neurovasc Res 9, 266–273.
PMID:22873723
- Impact of lipophilic antioxidants and level of antibodies against oxidized low-density lipoprotein in Polish children with phenylketonuria.
Mikoluc, B., Motkowski, R., Karpinska, J., Amilkiewicz, J., Didycz, B., Gizewska, M., Lange, A., Milanowski, A., Nowacka, M., Sands, D., Schneiberg, B., Starostecka, E., Wojcicka-Bartlomiejczyk, I., Piotrowska-Jastrzebska, J., 2012. Antioxid. Redox Signal. 16, 179–182
PMID:21895448
- Imaging of inflamed carotid artery atherosclerotic plaques with the use of 99mTc-HYNIC-IL-2 scintigraphy in end-stage renal disease patients.
Opalinska, M., Stompor, T., Pach, D., Mikolajczak, R., Fedak, D., Krzanowski, M., Rakowski, T., Sowa-Staszczak, A., Glowa, B., Garnuszek, P., Maurin, M., Karczmarczyk, U., Sulowicz, W., Hubalewska-Dydejczyk, A., 2012. European Journal of Nuclear Medicine and Molecular Imaging 39, 673–682.
- Lipid peroxidation markers in Crohn’s disease: the associations and diagnostic value.
Boehm, D., Krzystek-Korpacka, M., Neubauer, K., Matusiewicz, M., Paradowski, L., Gamian, A., 2012. Clin. Chem. Lab. Med. 50, 1359–1366.
PMID:22868800
- Oxidized LDL to autoantibodies against oxLDL ratio – The new biomarker associated with carotid atherosclerosis and cardiovascular complications in dialyzed patients.
Pawlak, K., Mysliwiec, M., Pawlak, D., 2012. Atherosclerosis 224, 252–257.
- On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm.
Pincemail, J., Defraigne, J.O., Cheramy-Bien, J.P., Dardenne, N., Donneau, A.F., Albert, A., Labropoulos, N., Sakalihasan, N., 2012. Redox Rep. 17, 139–144.
PMID:22732574
- Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction.
Turki, A., Hayot, M., Carnac, G., Pillard, F., Passerieux, E., Bommart, S., Raynaud de Mauverger, E., Hugon, G., Pincemail, J., Pietri, S., Lambert, K., Belayew, A., Vassetzky, Y., Juntas Morales, R., Mercier, J., Laoudj-Chenivesse, D., 2012. Free Radic. Biol. Med. 53, 1068–1079
PMID:22796148
- Association between moderately oxidized low-density lipoprotein and high-density lipoprotein particle subclass distribution in hemodialyzed and post-renal transplant patients.
Kimak, E., Hałabiś, M., Baranowicz-Gąszczyk, I., Solski, J., Książek, A., 2011. Journal of Zhejiang University SCIENCE B 12, 365–371
- The effect of olive oil polyphenols on antibodies against oxidized LDL. A randomized clinical trial.
Castañer, O., Fitó, M., López-Sabater, M.C., Poulsen, H.E., Nyyssönen, K., Schröder, H., Salonen, J.T., De la Torre-Carbot, K., Zunft, H.-F., De la Torre, R., Bäumler, H., Gaddi, A.V., Saez, G.T., Tomás, M., Covas, M.-I., EUROLIVE Study Group, 2011. Clin Nutr 30, 490–493
PMID:21376434
- Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Maes, M., Kubera, M., Uytterhoeven, M., Vrydags, N., Bosmans, E., 2011. Med. Sci. Monit. 17, SC11-15.
PMID:21455120; PMCID: PMC3539515
- Moderate consumption of red wine and human platelet responsiveness.
Tozzi Ciancarelli, M.G., Di Massimo, C., De Amicis, D., Ciancarelli, I., Carolei, A., 2011. Thromb. Res. 128, 124–129.
PMID:21489606
- Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease.
Maes, M., Mihaylova, I., Kubera, M., Uytterhoeven, M., Vrydags, N., Bosmans, E., 2010. J Affect Disord 125, 287–294.
PMID:20083310
- Associations between Oxidized LDL to LDL Ratio, HDL and Vascular Calcification in the Feet of Hemodialysis Patients.
An, W.S., Kim, S.-E., Kim, K.-H., Bae, H.-R., Rha, S.-H., 2009. Journal of Korean Medical Science 24, S115.
- Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study.
Bloomer, R.J., Larson, D.E., Fisher-Wellman, K.H., Galpin, A.J., Schilling, B.K., 2009. Lipids in Health and Disease 8, 36.
- Grape extract improves antioxidant status and physical performance in elite male athletes.
Lafay, S., Jan, C., Nardon, K., Lemaire, B., Ibarra, A., Roller, M., Houvenaeghel, M., Juhel, C., Cara, L., 2009 13
- Ethnic variation in levels of circulating IgG autoantibodies to oxidised low-density lipoprotein.
Miller, M.A., Strazzullo, P., Karanam, S., Cappuccio, F.P., 2009. Atherosclerosis 203, 126–136.
PMID:18606413
- Oxidized LDL and anti‐oxLDL antibody levels in peripheral atherosclerotic disease.
Andican, G., Seven, A., Uncu, M., Cantaşdemir, M., Numan, F., Burçak, G., 2008. Scandinavian Journal of Clinical and Laboratory Investigation 68, 473–478.
- Perceived work-related stress and early atherosclerotic changes in healthy employees.
Bugajska, J., Widerszal-Bazyl, M., Radkiewicz, P., Pasierski, T., Szulczyk, G.A., Ząbek, J., Wojciechowska, B., Jędryka-Góral, A., 2008. International Archives of Occupational and Environmental Health 81, 1037–1043.
- Oxidized low-density lipoproteins, autoantibodies against oxidized low-density lipoproteins and carotid intima media thickness in a clinically healthy population.
Chen, H.-W., Kuo, C.-L., Huang, C.-S., Kuo, S.-J., Liu, C.-S., 2008. Cardiology 110, 252–259.
PMID:18073481
- Effect of different contraceptive methods on the oxidative stress status in women aged 40 48 years from the ELAN study in the province of Liege, Belgium.
Pincemail, J., Vanbelle, S., Gaspard, U., Collette, G., Haleng, J., Cheramy-Bien, J.P., Charlier, C., Chapelle, J.P., Giet, D., Albert, A., Limet, R., Defraigne, J.O., 2007. Hum. Reprod. 22, 2335–2343.
PMID:17584753
- Effects of reconstituted HDL on charge-based LDL subfractions as characterized by capillary isotachophoresis.
Zhang, B., Uehara, Y., Hida, S., Miura, S., Rainwater, D.L., Segawa, M., Kumagai, K., Rye, K.-A., Saku, K., 2007. J. Lipid Res. 48, 1175–1189.
PMID:17327623
- Relationship of classical and non-classical risk factors with genetic variants relevant to coronary heart disease.
Manresa, J.M., Zamora, A., Tomás, M., Sentí, M., Fitó, M., Covas, M.I., Alcántara, M., Latorre, G., Escurriol, V., Domingues, S., Marrugat, J., 2006. Eur J Cardiovasc Prev Rehabil 13, 738–744.
PMID:17001213
- Possible association between circulating vascular endothelial growth factor and oxidative stress markers in hemodialysis patients. Pawlak, K., Pawlak, D., Myśliwiec, M., 2006. Med. Sci. Monit. 12, CR181-185.
PMID:16572054
- Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy.
Resch, U., Tatzber, F., Budinsky, A., Sinzinger, H., 2006. Br J Clin Pharmacol 61, 262–274.
PMID: 16487219; PMCID: PMC1885020
- Circulating oxidized low density lipoprotein, autoantibodies against them and homocysteine serum levels in diagnosis and estimation of severity of coronary artery disease.
Faviou, E., Vourli, G., Nounopoulos, C., Zachari, A., Dionyssiou-Asteriou, A., 2005. Free Radic. Res. 39, 419–429
PMID:16028367
- Evaluation of autoantibodies against oxidized LDL (oLAB) and blood antioxidant status in professional soccer players.
Kłapcińska, B., Kempa, K., Sobczak, A., Sadowska-Krepa, E., Jagsz, S., Szołtysek, I., 2005. Int J Sports Med 26, 71–78.
PMID:15643538
- Alteration of the Copy Number of Mitochondrial DNA in Leukocytes of Patients with Hyperlipidemia.
Liu, C.-S., Kuo, C.-L., Cheng, W.-L., Huang, C.-S., Lee, C.-F., Wei, Y.-H., 2005. Annals of the New York Academy of Sciences 1042, 70–75.
- Autoantibodies against oxidized low-density lipoprotein (ox-LDL) and LDL oxidation status.
Brizzi, P., Tonolo, G., Bertrand, G., Carusillo, F., Severino, C., Maioli, M., Malaguarnera, L., Musumeci, S., 2004. Clin. Chem. Lab. Med. 42, 164–170.
PMID:15061355
- The 161TT genotype in the exon 6 of the peroxisome-proliferator-activated receptor γ gene is associated with premature acute myocardial infarction and increased lipid peroxidation in habitual heavy smokers.
Chao, T.-H., Li, Y.-H., Chen, J.-H., Wu, H.-L., Shi, G.-Y., Liu, P.-Y., Tsai, W.-C., Guo, H.-R., 2004. Clinical Science 107, 461–466.
PMID:15217350
- Antibodies to oxidized low-density lipoprotein in patients following coronary artery revascularization.
Miller, E.R., Erlinger, T.P., Blumenthal, R.S., Margolis, S., Allen, J.K., 2003. Coron. Artery Dis. 14, 163–169.
PMID:12655280
- Antibodies against ox-LDL serum levels in patients with hepatocellular carcinoma. Motta, M., Pistone, G., Franzone, A.M., Romeo, M.A., Di Mauro, S., Giugno, I., Ruello, P., Malaguarnera, M., 2003. Panminerva Med 45, 69–73.
PMID:12682623
- The association of antibodies against oxidized low-density lipoprotein with atherosclerosis in hemodialysis patients.
Shoji, T., Kimoto, E., Shinohara, K., Emoto, M., Ishimura, E., Miki, T., Tsujimoto, Y., Tabata, T., Nishizawa, Y., 2003. Kidney Int. Suppl. S128-130.
PMID:12694327
- Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationships with oxidant-antioxidant system in patients with psoriasis. Vanizor Kural, B., Orem, A., Cimşit, G., Yandi, Y.E., Calapoglu, M., 2003. Clin. Chim. Acta 328, 71–82.
PMID:12559600
- The relationship between smoking habits and serum levels of 8-OHdG, oxidized LDL antibodies, Mn-SOD and carotenoids in rural Japanese residents.
Suzuki, K., Ito, Y., Ochiai, J., Aoki, K., Wakai, K., Tamakoshi, A., Ando, M., Watanabe, Y., Ozasa, K., Seki, N., Nishino, Y., Kondo, T., Ohno, Y., Tamakoshi, A., Mori, M., Motohashi, Y., Tsuji, I., Nakamura, Y., Iso, H., Mikami, H., Hashimoto, S., Inaba, Y., Hoshiyama, Y., Suzuki, H., Shimizu, H., Toyoshima, H., Tokudome, S., Ito, Y., Kikuchi, S., Koizumi, A., Kawamura, T., Watanabe, Y., Miki, T., Date, C., Sakata, K., Nose, T., Hayakawa, N., Yoshimura, T., Fukuda, K., Okamoto, N., Shio, H., Ohno, Y., Kitagawa, T., Kuroki, T., Tajima, K., Japan Collaborative Cohort Study Group, 2003. J Epidemiol 13, 29–37
PMID:12587611
- Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure.
Bennett-Richards, K.J., Kattenhorn, M., Donald, A.E., Oakley, G.R., Varghese, Z., Bruckdorfer, K.R., Deanfield, J.E., Rees, L., 2002. Kidney Int. 62, 1372–1378.
PMID:12234308
- Antibody to oxidized low-density lipoprotein and cardiovascular mortality in end-stage renal disease.
Shoji, T., Fukumoto, M., Kimoto, E., Shinohara, K., Emoto, M., Tahara, H., Koyama, H., Ishimura, E., Nakatani, T., Miki, T., Tsujimoto, Y., Tabata, T., Nishizawa, Y., 2002. Kidney Int. 62, 2230–2237.
PMID:12427150
- Decreased oxidative stress in patients with idiopathic dilated cardiomyopathy one year after immunoglobulin adsorption.
Schimke, I., Müller, J., Priem, F., Kruse, I., Schön, B., Stein, J., Kunze, R., Wallukat, G., Hetzer, R., 2001. Journal of the American College of Cardiology 38, 178–183.
- Acute and long-term effects of low-density lipoprotein (LDL)-apheresis on oxidative damage to LDL and reducing capacity of erythrocytes in patients with severe familial hypercholesterolaemia.
Stefanutti, C., Giacomo, S.D., Vivenzio, A., Isacchi, G.C., Masella, R., Caprari, P., Varì, R., Tarzia, A., Mosiello, A., Cantafora, A., 2001. Clinical Science 100, 191–198.
PMID:11171288
- Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects.
Shoji, T., Nishizawa, Y., Fukumoto, M., Shimamura, K., Kimura, J., Kanda, H., Emoto, M., Kawagishi, T., Morii, H., 2000. Atherosclerosis 148, 171–177.
- Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure.
Stenvinkel, P., Heimbürger, O., Paultre, F., Diczfalusy, U., Wang, T., Berglund, L., Jogestrand, T., 1999. Kidney Int. 55, 1899–1911.
PMID:10231453
- Autoantibodies against oxidated low density lipoproteins (oLAb) and procalcitonin (PCT) as prognostic markers for patients suffering from sepsis and systemic inflammatory response syndrome (SIRS).
Reiger, J., Tatzber, F., Ziervogel, G., Köller, U., Grimm, G., 1998. Crit Care 2, P006
- Lipid peroxidation parameters and antioxidant status of critically ill intensive care unit patients.
Smolle, K., Khoschsorur, G., Wonisch, W., Tatzber, F., 1998. Crit Care 2, P012.
 Download biomedica product list
Download biomedica product list