EZ4U – Cell Proliferation and Cytotoxicity Assay | BI-5000
-
Method
Cell proliferation and cytotoxicity assay, 10 x 96 tests, method based on the reduction of tetrazolium salt to colored formazan
-
Sample type
Cell culture
-
Sample volume
200 µl / test
-
Assay time
2 – 5 hours, depending on the metabolic capacity of the cells
-
Use
Research use only
EZ4U – Cell Proliferation Assay Product Overview
The EZ4U kit is a two to five-hour test for the measurement of cell proliferation and cell toxicity. The EZ4U test employs non-toxic tetrazolium salts, which are reduced to colored formazan. As this reduction process requires functional mitochondria, which are inactivated within a few minutes after cell death, this method provides an excellent tool for the discrimination between living and dead cells.
EZ4U – Cell Proliferation Assay Principle
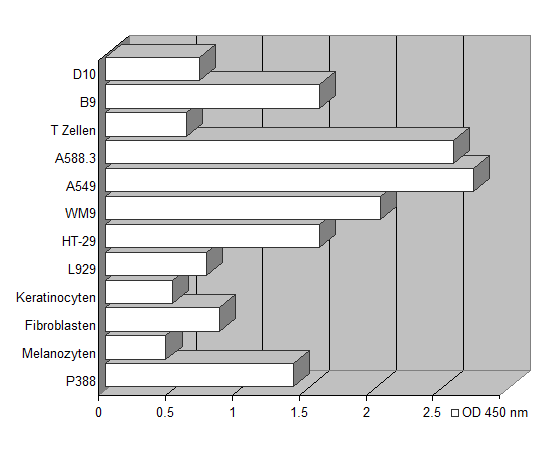
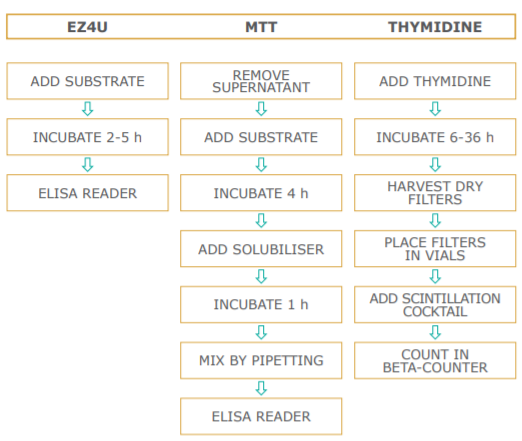
The assay set-up is performed in the same manner as the standard 3H-thymidine incorporation method. Instead of pulsing with tritiated nucleotide, 20 µl of dye solution is added to 200 µl sample. The incubation time is dependent on the metabolic capacity of the cells. Usually two to five hours of incubation at 37°C are sufficient to yield a significant increase in color intensity. As different cells vary in their ability to convert the yellow colored tetrazolium compound to its red formazan derivative, we recommend testing every new cell-line's metabolic capacity as described in the figure below.
Different metabolic capacity of various cell lines. 3 x 103 cells/well were cultivated in 200 µl RPMI 1640. Following a cultivation period of 3 days, 25 µl of the dye substrate were added to each well. Optical density was recorded after 4 hours, showing significant differences in the metabolic capacity of the various cell lines.
After incubation, the plate is removed from the incubator and gently mixed by tipping the plate towards all four sides. To avoid increased standard deviations, the plate must be shaken before reading the optical density.
The absorbance is measured on a microplate-reader set at 450 nm or 492 nm with 620 nm as a reference wavelength. The reference absorbance at 620 nm (or any wavelength between 620-690 nm) is used to correct for nonspecific background values caused by cell debris, fingerprints, or other potential interferences. However, the reference may be omitted without significant changes in the accuracy of the assay.
Method Comparison
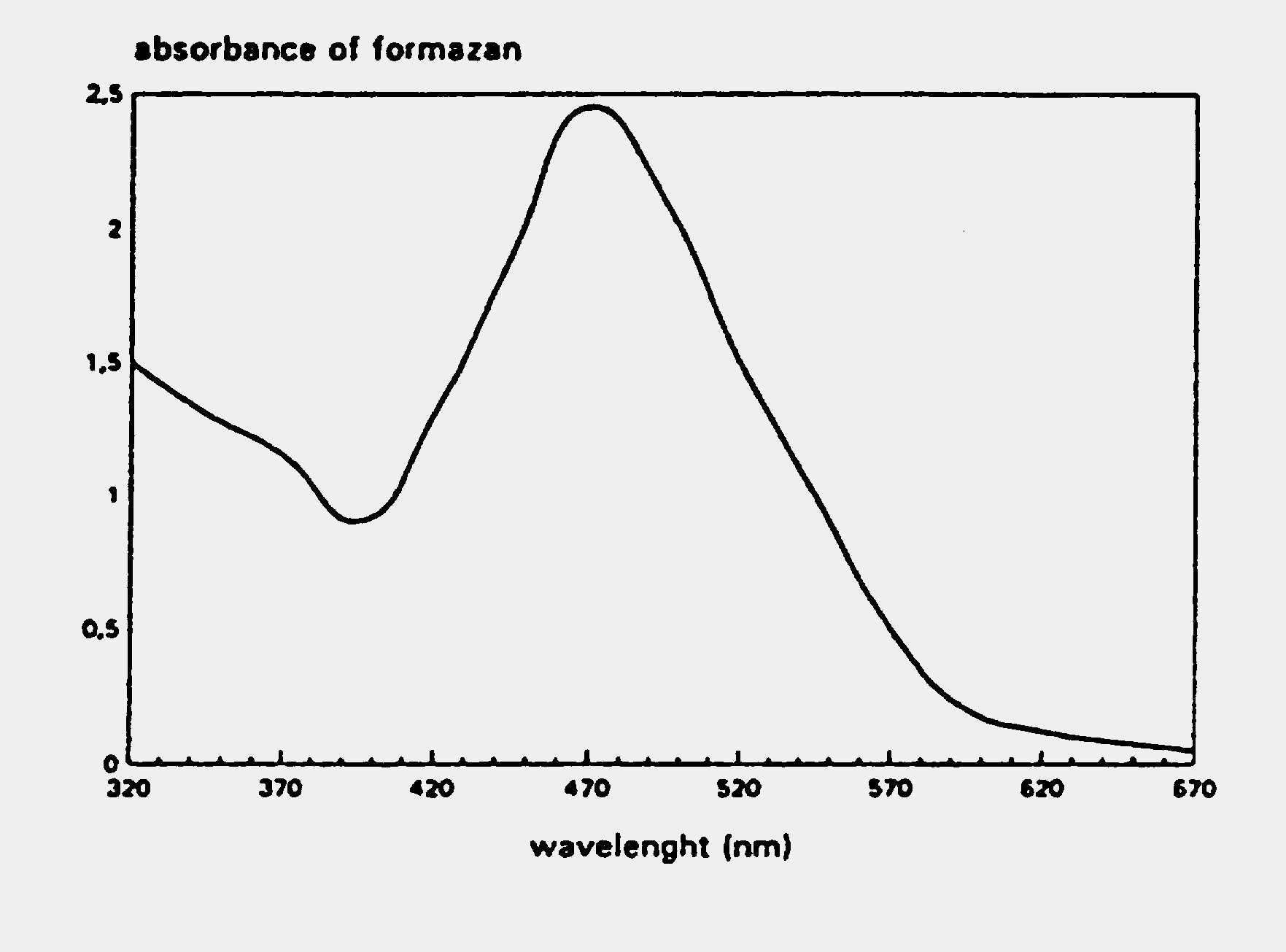
EZ4U – Cell Proliferation Assay Typical Absorption Spectrum
The figure below shows a typical absorption spectrum for the EZ4U assay.
EZ4U – Cell Proliferation Assay Kit Components
|
Contents |
Description |
Quantity |
|
SUB |
Substrate, lyophilized |
10 vials |
|
ACT |
Activator solution, ready to use |
1 x 30 ml |
|
|
Package insert + QC sheet |
1 piece each |
Storage instructions: All reagents of the EZ4U kit are stable at 4°C until the expiry date stated on the label of each reagent.
Reagent Preparation
One vial SUB contains enough substrate for 96 tests, i.e. one 96-well plate with 200 µl of cell culture medium/well. If more substrate is needed, combine the dissolved substrates prior to pulsing.
|
1. |
Dissolve the SUB (substrate) in 2.5 ml ACT (activator). |
|
2. |
Prewarm this solution to 37°C prior to addition. If necessary, warm up the substrate vial in your hand while mixing with activator. |
The mixed substrate is designed for immediate use only and should not be stored.
EZ4U – Cell Proliferation Assay Protocol
|
1. |
All reagents and samples must be at room temperature (18-26°C) before use in the assay. |
|
2. |
Add 200 µl cell culture into the respective wells. |
|
3. |
Add 20 µl SUB (substrate) into each well, swirl gently. |
|
4. |
Incubate at 37°C for 2-5 hours dependent on the metabolic capacity of the cells. |
|
5. |
If no microplate reader with a shaking plate carrier is available, mix the plate on a vibrating platform or by tipping it by hand. |
|
6. |
Read absorbance at 450 nm or 492 nm, with 620 nm as reference. |
|
7. |
To improve the accuracy of the results, absorbance from a substrate blank in assay medium without cells should be subtracted from all other values. |
Important Considerations
|
The substrate is not sterile. If sterile conditions are demanded, the solubilised ready-to-use dye substrate must be sterile filtered. (A minor turbidity prior to filtration interferes neither with the filtration, nor with the assay performance). |
|
Due to the high sensitivity of this test, it is advisable to use as few cells as possible. Otherwise a non-linear titration curve may result. |
|
To achieve reproducible time kinetics in color development, equilibrate cell cultures at 37°C. |
|
Do not prolong incubation times without prior testing, as this might result in an increased background without improved sensitivity. |
|
The use of a reference wavelength of 620 nm (which is subtracted from the values obtained at 450 or 492 nm) is not strictly necessary but increases the performance of the test. |
|
The chromophore appears to be non-toxic and therefore continuation of cell culture is possible after the removal of the formazan derivative. |
Technical Hints
|
Do not mix or substitute reagents with those from other lots or sources. |
|
Do not mix stoppers and caps from different reagents or use reagents between lots. |
|
Do not use reagents beyond expiration date. |
|
Protect reagents from direct sunlight. |
|
Avoid foaming when mixing reagents. |
Proliferation assays are widely used in cell biology for the study of growth factors, cytokines, nutrients and for the screening of cytotoxic or chemotherapeutic agents. There are several ways to determine the number of cells either by microscopic inspection, through an electronic particle counter, indirectly by measuring the incorporation of radioactive precursors, quantifying total protein with chromogenic dyes, or by measuring metabolic activity of cellular enzymes. The most common assay for cell proliferation is the incorporation of 3H‑thymidine into cellular DNA. The 3H-thymidine assay is, however, labor-intensive as it requires the removal of the excess, unincorporated label by using some cell harvesting method before measurement. In 1956, the first paper on the use of tetrazolium salts as indicators of cell viability was published. The method was based on the finding that living cells are capable of reducing slightly colored or uncolored tetrazolium salts into intensely colored formazan derivatives. This reduction process requires functional mitochondria, which are inactivated within a few minutes of cell death. Therefore, this method provides an excellent tool for the discrimination of living and dead cells. However, the early tetrazolium salts did have some disadvantages, such as the insolubility of the resulting formazan products. Time and labor-consuming solubilization procedures were necessary, including repetitive pipetting and mixing, or the application of hazardous solubilizers. This necessary post-assay treatment, however, irreversibly terminated cell proliferation and thus made it impossible to prolong incubation in order to achieve an increase in sensitivity or continue cell culture. These inconveniences led to the development of non-toxic tetrazolium salts which yield soluble reduction products. Although the assay procedure was made easier by these soluble dyes, in practice the use was limited due to the instability of the formazan dye and a relatively low absorbance of the end product as compared to the classical MTT assay.
The Biomedica research department has solved both problems and created an easy-to-use, rapid and reliable non-isotopic cell proliferation assay. For convenience, we have made it highly compatible with the standard thymidine incorporation assay. Therefore, no changes are required in the setup of the test and in the "labeling" procedure. Furthermore, there is no need for the removal of culture medium before or after the addition of the chromogenic substrate and neither solubilization nor harvesting procedures are necessary. The work performed by Biomedica resulted in an assay which combines the best of the thymidine and MTT methods, namely: accuracy, speed, reliability, and ease of use. Also, according to our data achieved so far, the chromophore appears to be non-toxic. A double labeling with EZ4U and a radioactive nucleotide to obtain more information about cell viability and DNA content is now feasible.
- Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea. Labuda R, Bacher M, Gratzl H, Doppler M, Parich A, Aufy M, Lemmens-Gruber R, Schuhmacher R, Rychli K, Wagner M, Rosenau T, Strauss J, Schüller C. Molecules. 2021 Oct 30;26(21):6589. doi: 10.3390/molecules26216589. PMID: 34770997.
- On the Biocompatibility and Teat Retention of In Situ Gelling Intramammary Formulations: Cattle Mastitis Prevention and Treatment. Pharmaceutics. Bhattarai S, Perumal D, Rathbone MJ, Bunt CR, Alany RG. 2021 Oct 19;13(10):1732. PMID: 34684025; PMCID: PMC8539992.
- Liposomal formulations of anticancer copper(II) thiosemicarbazone complexes. Mathuber M, Hager S, Keppler BK, Heffeter P, Kowol CR. Dalton Trans. 2021 Oct 7. doi: 10.1039/d1dt02763h. Epub ahead of print. PMID: 34617075
- Influence of Human Jaw Periosteal Cells Seeded β-Tricalcium Phosphate Scaffolds on Blood Coagulation. Weber M, Umrath F, Steinle H, Schmitt LF, Yu LT, Schlensak C, Wendel HP, Reinert S, Alexander D, Avci-Adali M. Int J Mol Sci. 2021 Sep 14;22(18):9942. PMID: 34576103; PMCID: PMC8467579.
- Tolerability to non-endosomal, micron-scale cell penetration probed with magnetic particles. Ruiz-Cánovas E, Mendoza R, Villaverde A, Corchero JL.Colloids Surf B Biointerfaces. 2021 Sep 20;208:112123. PMID: 34571468.
- Anti-Oxidative and Immune Regulatory Responses of THP-1 and PBMC to Pulsed EMF Are Field-Strength Dependent. Int J Environ Res Public Health. Groiss S, Lammegger R, Brislinger D. 2021 Sep 9;18(18):9519. PMID: 34574442; PMCID: PMC8471206.
- Interactions of BRCA1-mutated Breast Cancer Cell Lines with Adipose-derived Stromal Cells (ADSCs).Plangger A, Haslik W, Rath B, Neumayer C, Hamilton G.J Mammary Gland Biol Neoplasia. 2021 Sep;26(3):235-245. PMID: 34228231.
- Structure-Activity Relationships of Triple-Action Platinum(IV) Prodrugs with Albumin-Binding Properties and Immunomodulating Ligands. Fronik P, Poetsch I, Kastner A, Mendrina T, Hager S, Hohenwallner K, Schueffl H, Herndler-Brandstetter D, Koellensperger G, Rampler E, Kopecka J, Riganti C, Berger W, Keppler BK, Heffeter P, Kowol CR.J Med Chem. 2021 Aug 26;64(16):12132-12151. PMID: 34403254; PMCID: PMC8404199.
- Effects of Metformin on Bone-derived Mesenchymal Stromal Cell–breast Cancer Cell Line Interactions . Teufelsbauer M, Lang C, Plangger A et al. 2021 Aug 5. Preprint (Version 1) available at Research Square.
- New Hybrid Compounds Combining Fragments of Usnic Acid and Monoterpenoids for Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibition. Dyrkheeva NS, Filimonov AS, Luzina OA, Zakharenko AL, Ilina ES, Malakhova AA, Medvedev SP, Reynisson J, Volcho KP, Zakian SM, Salakhutdinov NF, Lavrik OI. Biomolecules. 2021 Jul 1;11(7):973. PMID: 34356597; PMCID: PMC8301776.
- Anti-inflammatory effects of two lupane-type triterpenes from leaves of Acanthopanax gracilistylus on LPS-induced RAW264.7 macrophages. Jiao LUO, Xiao-jun LI and Geon-ho LEE et al. Food Science and Technology. DOI: 10.1590/fst.89721.
- Antimicrobial Activity of Quasi-Enantiomeric Cinchona Alkaloid Derivatives and Prediction Model Developed by Machine Learning. Antibiotics (Basel). Ramić A, Skočibušić M, Odžak R, Čipak Gašparović A, Milković L, Mikelić A, Sović K, Primožič I, Hrenar T. 2021 May 31;10(6):659. PMID: 34073082; PMCID: PMC8229948.
- Complementary Omics Strategies to Dissect p53 Signaling Networks Under Nutrient Stress. Galhuber M, Michenthaler H, Heininger C et al., Papers, SSRN.
- Knockdown of the mRNA encoding the ribosomal protein eL38 in mammalian cells causes a substantial reorganization of genomic transcription. Gopanenko AV, Kolobova AV, Meschaninova MI, Venyaminova AG, Tupikin AE, Kabilov MR, Malygin AA, Karpova GG. Biochimie. 2021 May;184:132-142. PMID: 33675855.
- Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments. Molecules. Munkuev AA, Mozhaitsev ES, Chepanova AA, Suslov EV, Korchagina DV, Zakharova OD, Ilina ES, Dyrkheeva NS, Zakharenko AL, Reynisson J, Volcho KP, Salakhutdinov NF, Lavrik OI. 2021 May 24;26(11):3128. PMID: 34073771; PMCID: PMC8197275.
- Contribution of Syndecans to the Cellular Entry of SARS-CoV-2. Hudák A, Letoha A, Szilák L, Letoha T. Int J Mol Sci. 2021 May 19;22(10):5336. PMID: 34069441; PMCID: PMC8159090.
- Tackling resistance in chronic myeloid leukemia: Novel cell death modulators with improved efficacy. Schoepf AM, Salcher S, Obexer P, Gust R. Eur J Med Chem. 2021 Apr 15;216:113285. PMID: 33662676.
- Zinc Chloride: Time-Dependent Cytotoxicity, Proliferation and Promotion of Glycoprotein Synthesis and Antioxidant Gene Expression in Human Keratinocytes. Salesa, B.; Sabater i Serra, R.; Serrano-Aroca, Á. Biology 2021, 10, 1072.
- Regular Exercise May Restore Certain Age-Related Alterations of Adaptive Immunity and Rebalance Immune Regulation. Front Immunol. Papp G, Szabó K, Jámbor I, Mile M, Berki AR, Arany AC, Makra G, Szodoray P, Csiki Z, Balogh L. 2021 Apr 16;12:639308. PMID: 33936054; PMCID: PMC8085426.
- The Dysregulated Galectin Network Activates NF-κB to Induce Disease Markers and Matrix Degeneration in 3D Pellet Cultures of Osteoarthritic Chondrocytes. Pichler KM, Weinmann D, Schmidt S, Kubista B, Lass R, Martelanz L, Alphonsus J, Windhager R, Gabius HJ, Toegel S. Calcif Tissue Int. 2021 Mar;108(3):377-390. PMID: 33185768; PMCID: PMC7881967.
- Discovery of Novel Sultone Fused Berberine Derivatives as Promising Tdp1 Inhibitors. Molecules. Gladkova ED, Chepanova AA, Ilina ES, Zakharenko AL, Reynisson J, Luzina OA, Volcho KP, Lavrik OI, Salakhutdinov NF.2021 Mar 30;26(7):1945. PMID: 33808389; PMCID: PMC8037669.
- Role of VDR in stellate cells activation and tumor-stroma crosstalk in pancreatic cancer. Wu, Yang (2021):Dissertation, LMU München: Faculty of Medicine.
- Fasting reverses drug-resistance in hepatocellular carcinoma through p53-dependent metabolic synergism. Krstic, J., Reinisch, I., Schindlmaier, K., Galhuber, M., Berger, N., Kupper, Net al. 2021. Cold Spring Harbor Laboratory; 2021 Feb 11. bioRxiv.
- Uncovering molecular mechanisms of regulated cell death in the naked mole rat. Evdokimov A, Popov A, Ryabchikova E, Koval O, Romanenko S, Trifonov V, Petruseva I, Lavrik I, Lavrik O. Aging (Albany NY). 2021 Jan 28;13(3):3239-3253. PMID: 33510044; PMCID: PMC7906159.
- Eosinophils adhesion assay as a tool for phenotypic drug screening - The pharmacology of 1,3,5 - Triazine and 1H-indole like derivatives against the human histamine H4 receptor. Grosicki M, Adami M, Micheloni C, Głuch-Lutwin M, Siwek A, Latacz G, Łażewska D, Więcek M, Reiner-Link D, Stark H, Chlopicki S, Kieć-Kononowicz K. Eur J Pharmacol. 2021 Jan 5;890:173611. PMID: 33017589.
- Sensitivity of Osteosarcoma Cells to Concentration-Dependent Bioactivities of Lipid Peroxidation Product 4-Hydroxynonenal Depend on Their Level of Differentiation. Sunjic SB, Gasparovic AC, Jaganjac M, Rechberger G, Meinitzer A, Grune T, Kohlwein SD, Mihaljevic B, Zarkovic N. Cells. 2021 Jan 29;10(2):269. PMID: 33572933; PMCID: PMC7912392.
- Eosinophils adhesion assay as a tool for phenotypic drug screening - The pharmacology of 1,3,5 - Triazine and 1H-indole like derivatives against the human histamine H4 receptor. Grosicki M, Adami M, Micheloni C, Głuch-Lutwin M, Siwek A, Latacz G, Łażewska D, Więcek M, Reiner-Link D, Stark H, Chlopicki S, Kieć-Kononowicz K. Eur J Pharmacol. 2021 Jan 5;890:173611. PMID: 33017589.
- Sensitivity of Osteosarcoma Cells to Concentration-Dependent Bioactivities of Lipid Peroxidation Product 4-Hydroxynonenal Depend on Their Level of Differentiation. Sunjic SB, Gasparovic AC, Jaganjac M, Rechberger G, Meinitzer A, Grune T, Kohlwein SD, Mihaljevic B, Zarkovic N. Cells. 2021 Jan 29;10(2):269. PMID: 33572933; PMCID: PMC7912392.
- An insight of techniques for the assessment of permeation flux across the skin for optimization of topical and transdermal drug delivery systems. Shashank Chaturvedi, Anuj Garg. Journal of Drug Delivery Science and Technology. 2021, 62; 102355.
- Cytotoxicity of combinations of the pan-KRAS inhibitor BAY-293 against primary non-small lung cancer cells. Plangger A, Rath B, Hochmair M, Funovics M, Hamilton G. Transl Oncol. 2021 Dec;14(12):101230. PMID: 34598083; PMCID: PMC8488304.
- The vitamin D analogue calcipotriol promotes an anti-tumorigenic phenotype of human pancreatic CAFs but reduces T cell mediated immunity. Gorchs L, Ahmed S, Mayer C, Knauf A, Fernández Moro C, Svensson M, Heuchel R, Rangelova E, Bergman P, Kaipe H Sci Rep. 2020 Oct 15;10(1):17444. doi: 10.1038/s41598-020-74368-3. PMID: 33060625; PMCID: PMC7562723.
- Synthesis, characterization and biological activity of bromido[3-ethyl-4-aryl-5-(2-methoxypyridin-5-yl)-1-propyl-1,3-dihydro-2H-imidazol-2-ylidene]gold(i) complexes. Gallati CM , Goetzfried SK , Ausserer M , Sagasser J , Plangger M , Wurst K , Hermann M , Baecker D , Kircher B , Gust R Dalton Trans. 2020 May 7;49(17):5471-5481. PMID: 32255443.
-
Lipid droplet-mediated scavenging as novel intrinsic and adaptive resistance factor against the multikinase inhibitor ponatinib. Englinger, B., Laemmerer, A., Moser, P., Kallus, S., Röhrl, C., Pirker, C., Baier, D., Mohr, T., Niederstaetter, L., Meier‐Menches, S.M., Gerner, C., Gabler, L., Gojo, J., Timelthaler, G., Senkiv, J., Jäger, W., Kowol, C.R., Heffeter, P., Berger, W., 2020. International Journal of Cancer.
- Characteristics of Hyaluronan Synthesis Inhibition by 4-Methylumbelliferone in Orbital Fibroblasts. Galgoczi, E., Jeney, F., Katko, M., Erdei, A., Gazdag, A., Sira, L., Bodor, M., Berta, E., Ujhelyi, B., Steiber, Z., Gyory, F., Nagy, E.V., 2020. Invest. Ophthalmol. Vis. Sci. 61, 27–27.
- E-cigarettes: Effects in phagocytosis and cytokines response against Mycobacterium tuberculosis. Gómez, A.-C., Rodríguez-Fernández, P., Villar-Hernández, R., Gibert, I., Muriel-Moreno, B., Lacoma, A., Prat-Aymerich, C., Domínguez, J., 2020. PLOS ONE 15, e0228919.
- Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation. Moschovaki Filippidou, F., Kirsch, A.H., Thelen, M., Kétszeri, M., Artinger, K., Aringer, I., Schabhüttl, C., Mooslechner, A.A., Frauscher, B., Pollheimer, M., Niedrist, T., Meinitzer, A., Drucker, D.J., Pieber, T.R., Eller, P., Rosenkranz, A.R., Heinemann, A., Eller, K., 2020. The American Journal of Pathology 190, 400–411.
- Investigation of The Cellular Response to Bone Fractures: Evidence for Flexoelectricity. Núñez-Toldrà, R., Vasquez-Sancho, F., Barroca, N., Catalan, G., 2020. Scientific Reports 10, 1–10.
- Establishment of Collagen: Hydroxyapatite/BMP-2 Mimetic Peptide Composites. Schuster, L., Ardjomandi, N., Munz, M., Umrath, F., Klein, C., Rupp, F., Reinert, S., Alexander, D., 2020. Materials 13, 1203.
- Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Vater, C., Hlawaty, V., Werdenits, P., Cichoń, M.A., Klang, V., Elbe-Bürger, A., Wirth, M., Valenta, C., 2020. International Journal of Pharmaceutics 580, 119209.
- Cell-Type Specific Metabolic Response of Cancer Cells to Curcumin. Mojzeš, A., Tomljanović, M., Milković, L., Novak Kujundžić, R., Čipak Gašparović, A., Gall Trošelj, K., 2020. Int J Mol Sci 21. PMID: 32121279; PMCID: PMC7084320
- iPSC-Derived MSCs Versus Originating Jaw Periosteal Cells: Comparison of Resulting Phenotype and Stem Cell Potential. Umrath, F., Weber, M., Reinert, S., Wendel, H.-P., Avci-Adali, M., Alexander, D., 2020. Int J Mol Sci 21.
- Solution equilibrium, structural and cytotoxicity studies on Ru(η6-p-cymene) and copper complexes of pyrazolyl thiosemicarbazones. Dömötör, O., Kiss, M.A., Gál, G.T., May, N.V., Spengler, G., Nové, M., Gašparović, A.Č., Frank, É., Enyedy, É.A., 2020. Journal of Inorganic Biochemistry 202, 110883.
- Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Gerhartl, A., Hahn, K., Neuhoff, A., Friedl, H.-P., Förster, C.Y., Wunder, C., Schick, M., Burek, M., Neuhaus, W., 2020. Brain Research 1727, 146560.
- Chronic Oxidative Stress Promotes Molecular Changes Associated with Epithelial Mesenchymal Transition, NRF2, and Breast Cancer Stem Cell Phenotype. Čipak Gašparović, A., Milković, L., Dandachi, N., Stanzer, S., Pezdirc, I., Vrančić, J., Šitić, S., Suppan, C., Balic, M., 2019. Antioxidants 8, 633.
- Chapter 9 - Sol-gel composites based on alumina and ferria for cardiovascular diseases treatment. Drozdov, A.S., Fakhardo, A.F., Vinogradov, V.V., 2019, in: Melnyk, I.V., Vaclavikova, M., Seisenbaeva, G.A., Kessler, V.G. (Eds.), Biocompatible Hybrid Oxide Nanoparticles for Human Health, Micro and Nano Technologies. Elsevier, pp. 149–179.
- Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Gyöngyösi, M., Lukovic, D., Zlabinger, K., Spannbauer, A., Gugerell, A., Pavo, N., Traxler, D., Pils, D., Maurer, G., Jakab, A., Riesenhuber, M., Pircher, A., Winkler, J., Bergler-Klein, J., 2019. Cardiovascular Research Epub ahead of print. PMID: 31346605
- Effects of salinomycin and niclosamide on small cell lung cancer and small cell lung cancer circulating tumor cell lines. Hochmair, M., Rath, B., Klameth, L., Ulsperger, E., Weinlinger, C., Fazekas, A., Plangger, A., Zeillinger, R., Hamilton, G., 2019. Invest New Drugs.
- Sulfonamide-based diffusible signal factor analogs interfere with quorum sensing in Stenotrophomonas maltophilia and Burkholderia cepacia. Huedo, P., Kumar, V.P., Horgan, C., Yero, D., Daura, X., Gibert, I., O’Sullivan, T.P., 2019. Future Medicinal Chemistry 11, 1565–1582.
- Safety and Toxicity Counts of Nanocosmetics. Jeswani, G., Das Paul, S., Chablani, L., Ajazuddin, 2019, in: Cornier, J., Keck, C.M., Van de Voorde, M. (Eds.), Nanocosmetics: From Ideas to Products. Springer International Publishing, Cham, pp. 299–335.
- Radiosensitizing effects of trabectedin on human A549 lung cancer cells and HT-29 colon cancer cells. Manda, K., Präkelt, T., Schröder, T., Kriesen, S., Hildebrandt, G., 2019. Invest New Drugs.
- Nutritional Stress in Head and Neck Cancer Originating Cell Lines: The Sensitivity of the NRF2-NQO1 Axis. Milković, L., Tomljanović, M., Čipak Gašparović, A., Novak Kujundžić, R., Šimunić, D., Konjevoda, P., Mojzeš, A., Đaković, N., Žarković, N., Gall Trošelj, K., 2019. Cells 8, 1001.
- Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation. Moschovaki Filippidou, F., Kirsch, A.H., Thelen, M., Kétszeri, M., Artinger, K., Aringer, I., Schabhüttl, C., Mooslechner, A.A., Frauscher, B., Pollheimer, M., Niedrist, T., Meinitzer, A., Drucker, D.J., Pieber, T.R., Eller, P., Rosenkranz, A.R., Heinemann, A., Eller, K., 2019. The American Journal of Pathology.
- Critical Impact of Human Amniotic Membrane Tension on Mitochondrial Function and Cell Viability In Vitro. Poženel, L., Lindenmair, A., Schmidt, K., Kozlov, A.V., Grillari, J., Wolbank, S., Banerjee, A., Weidinger, A., 2019. Cells 8, 1641.
- Lipid Profile and Aquaporin Expression under Oxidative Stress in Breast Cancer Cells of Different Malignancies [WWW Document]. Rodrigues, C., Milkovic, L., Bujak, I.T., Tomljanovic, M., Soveral, G., Cipak Gasparovic, A., 2019. Oxidative Medicine and Cellular Longevity.
- Combined chemosensitivity and chromatin profiling prioritizes drug combinations in CLL. Schmidl, C., Vladimer, G.I., Rendeiro, A.F., Schnabl, S., Krausgruber, T., Taubert, C., Krall, N., Pemovska, T., Araghi, M., Snijder, B., Hubmann, R., Ringler, A., Runggatscher, K., Demirtas, D., de la Fuente, O.L., Hilgarth, M., Skrabs, C., Porpaczy, E., Gruber, M., Hoermann, G., Kubicek, S., Staber, P.B., Shehata, M., Superti-Furga, G., Jäger, U., Bock, C., 2019. Nat Chem Biol 15, 232–240.
- TRAIL-Receptor 4 Modulates γδ T Cell-Cytotoxicity Toward Cancer Cells. Tawfik, D., Groth, C., Gundlach, J.-P., Peipp, M., Kabelitz, D., Becker, T., Oberg, H.-H., Trauzold, A., Wesch, D., 2019. Front. Immunol. 10.
- LRPAP1 is a frequent proliferation-inducing antigen of BCRs of mantle cell lymphomas and can be used for specific therapeutic targeting. Thurner, L., Hartmann, S., Fadle, N., Kemele, M., Bock, T., Bewarder, M., Regitz, E., Neumann, F., Nimmesgern, A., Müller, L. von, Pott, C., Kim, Y.-J., Bohle, R.M., Wasik, M., Schuster, S.J., Hansmann, M.-L., Preuss, K.-D., Pfreundschuh, M., 2019. Leukemia 33, 148–158.
- Pixantrone demonstrates significant in vitro activity against multiple myeloma and plasma cell leukemia. Willenbacher, E., Jöhrer, K., Willenbacher, W., Flögel, B., Greil, R., Kircher, B., 2019. Ann Hematol 98, 2569–2578.
- Synergetic Effect of EP1 Receptor Antagonist and (-)-Epigallocatechin-3-gallate in Hepatocellular Carcinoma. Yang, H., Wang, M., Sun, H., Zhu, S., Jin, J., 2019. PHA 104, 267–275. PMID: 31434088
- Matryoshka-type gastro-resistant microparticles for the oral treatment of Mycobacterium tuberculosis.
Andreu, V., Larrea, A., Rodriguez-Fernandez, P., Alfaro, S., Gracia, B., Lucía, A., Usón, L., Gomez, A.-C., Mendoza, G., Lacoma, A., Dominguez, J., Prat, C., Sebastian, V., Ainsa, J.A., Arruebo, M., 2019. Nanomedicine (Lond) 14, 707–726.
PMID:30734643
- Kynurenic Acid and Its Analogs Are Beneficial Physiologic Attenuators in Bdelloid Rotifers.
Datki, Z., Galik-Olah, Z., Bohar, Z., Zadori, D., Fulop, F., Szatmari, I., Galik, B., Kalman, J., Vecsei, L., 2019. Molecules 24, 2171.
- Position-Selective Synthesis and Biological Evaluation of Four Isomeric A-Ring Amino Derivatives of the Alkaloid Luotonin A.
Ibric, A., Eckerstorfer, S., Eder, M., Louko, I., Tunjic, L., Heffeter, P., Schueffl, H.H., Marian, B., Haider, N., 2019. Molecules 24.
PMID:30781470; PMCID: PMC6412769
- Synthesis, Characterization and in vitro Studies of a Cathepsin B‐Cleavable Prodrug of the VEGFR Inhibitor Sunitinib.
Karnthaler‐Benbakka, C., Koblmüller, B., Mathuber, M., Holste, K., Berger, W., Heffeter, P., Kowol, C.R., Keppler, B.K., 2019. Chem Biodivers 16.
PMID:30566287; PMCID: PMC6391952
- Patient-derived cell line models revealed therapeutic targets and molecular mechanisms underlying disease progression of high grade serous ovarian cancer.
Kreuzinger, C., von der Decken, I., Wolf, A., Gamperl, M., Koller, J., Karacs, J., Pfaffinger, S., Bartl, T., Reinthaller, A., Grimm, C., Singer, C.F., Braicu, E.I., Cunnea, P., Gourley, C., Smeets, D., Boeckx, B., Lambrechts, D., Perco, P., Horvat, R., Berns, E.M.J.J., Cacsire Castillo-Tong, D., 2019. Cancer Lett. 459, 1–12.
PMID:31150822
- Targeted delivery and endosomal cellular uptake of DARPin-siRNA bioconjugates: Influence of linker stability on gene silencing.
Lorenzer, C., Streußnig, S., Tot, E., Winkler, A.-M., Merten, H., Brandl, F., Sayers, E.J., Watson, P., Jones, A.T., Zangemeister-Wittke, U., Plückthun, A., Winkler, J., 2019. European Journal of Pharmaceutics and Biopharmaceutics 141, 37–50.
- Surface functionalization via PEO coating and RGD peptide for nanostructured titanium implants and their in vitro assessment.
Parfenov, E.V., Parfenova, L.V., Dyakonov, G.S., Danilko, K.V., Mukaeva, V.R., Farrakhov, R.G., Lukina, E.S., Valiev, R.Z., 2019. Surface and Coatings Technology 357, 669–683.
- Combined chemosensitivity and chromatin profiling prioritizes drug combinations in CLL.
Schmidl, C., Vladimer, G.I., Rendeiro, A.F., Schnabl, S., Krausgruber, T., Taubert, C., Krall, N., Pemovska, T., Araghi, M., Snijder, B., Hubmann, R., Ringler, A., Runggatscher, K., Demirtas, D., de la Fuente, O.L., Hilgarth, M., Skrabs, C., Porpaczy, E., Gruber, M., Hoermann, G., Kubicek, S., Staber, P.B., Shehata, M., Superti-Furga, G., Jäger, U., Bock, C., 2019. Nat Chem Biol 15, 232–240.
- A Preclinical Model for Studying Herpes Simplex Virus Infection.
Tajpara, P., Mildner, M., Schmidt, R., Vierhapper, M., Matiasek, J., Popow-Kraupp, T., Schuster, C., Elbe-Bürger, A., 2019. Journal of Investigative Dermatology 139, 673–682.
- Cytotoxicity of lecithin-based nanoemulsions on human skin cells and ex vivo skin permeation: Comparison to conventional surfactant types.
Vater, C., Adamovic, A., Ruttensteiner, L., Steiner, K., Tajpara, P., Klang, V., Elbe-Bürger, A., Wirth, M., Valenta, C., 2019. International Journal of Pharmaceutics 566, 383–390.
-
Alencar, N., Sola, I., Linares, M., Juárez-Jiménez, J., Pont, C., Viayna, A., Vílchez, D., Sampedro, C., Abad, P., Pérez-Benavente, S., Lameira, J., Bautista, J.M., Muñoz-Torrero, D., Luque, F.J., 2018. Eur J Med Chem 146:108–122.
PMID:29407943
-
Apfelthaler, C., Skoll, K., Ciola, R., Gabor, F., Wirth, M., 2018. Eur J Pharm Biopharm 130:177–184.
PMID:29960015
-
Brislinger, D., Daxböck, C., Roßmanith, E., Stückler, M., Lang, I., Falkenhagen, D., 2018. J Ethnopharmacol 225:309–318.
PMID:30036577
-
Exceptional in vivo catabolism of neurodegeneration-related aggregates.
Datki, Z., Olah, Z., Hortobagyi, T., Macsai, L., Zsuga, K., Fulop, L., Bozso, Z., Galik, B., Acs, E., Foldi, A., Szarvas, A., Kalman, J., 2018. Acta Neuropathologica Communications 6:6.
-
Protein nanoparticles are nontoxic, tuneable cell stressors.
de Pinho Favaro, M.T., Sánchez-García, L., Sánchez-Chardi, A., Roldán, M., Unzueta, U., Serna, N., Cano-Garrido, O., Azzoni, A.R., Ferrer-Miralles, N., Villaverde, A., Vázquez, E., 2018. Nanomedicine (Lond) 13(3):255–268.
PMID:29338574
-
Dufour, A.M., Alvarez, M., Russo, B., Chizzolini, C., 2018. Front Immunol 9, 1865.
PMID:30150989; PMCID: PMC6099180
-
Interleukin-4 induces a CD44high /CD49bhigh PC3 subpopulation with tumor-initiating characteristics.
Erb, H.H.H., Guggenberger, F., Santer, F.R., Culig, Z., 2018. J. Cell. Biochem. 119(5):4103–4112.
PMID:29236307; PMCID: PMC5900863
-
Naked mole rat cells display more efficient excision repair than mouse cells.
Evdokimov, A., Kutuzov, M., Petruseva, I., Lukjanchikova, N., Kashina, E., Kolova, E., Zemerova, T., Romanenko, S., Perelman, P., Prokopov, D., Seluanov, A., Gorbunova, V., Graphodatsky, A., Trifonov, V., Khodyreva, S., Lavrik, O., 2018. Aging. 10(6):1454–1473.
PMID:29930219; PMCID: PMC6046242
-
Heilos, D., Röhrl, C., Pirker, C., Englinger, B., Baier, D., Mohr, T., Schwaiger, M., Iqbal, S.M., van Schoonhoven, S., Klavins, K., Eberhart, T., Windberger, U., Taibon, J., Sturm, S., Stuppner, H., Koellensperger, G., Dornetshuber-Fleiss, R., Jäger, W., Lemmens-Gruber, R., Berger, W., 2018. Oncotarget 9:25661–25680.
PMID:29876015; PMCID: PMC5986646
-
Comb-like PEG-containing polymeric composition as low toxic drug nanocarrier.
Kobylinska, L., Patereha, I., Finiuk, N., Mitina, N., Riabtseva, A., Kotsyumbas, I., Stoika, R., Zaichenko, A., Vari, S.G., 2018. Cancer Nanotechnology 9:11.
-
Latacz, G., Lubelska, A., Jastrzębska-Więsek, M., Partyka, A., Kucwaj-Brysz, K., Wesołowska, A., Kieć-Kononowicz, K., Handzlik, J., 2018. Bioorg. Med. Chem. Lett. 28(5):878–883.
PMID:29439902
-
Milkovic, L., Vukovic, T., Zarkovic, N., Tatzber, F., Bisenieks, E., Kalme, Z., Bruvere, I., Ogle, Z., Poikans, J., Velena, A., Duburs, G., 2018. Antioxidants 7(9):123.
PMID:30235855; PMCID: PMC6162383
-
Molter, A., Kathrein, S., Kircher, B., Mohr, F., 2018. Dalton Trans 47:5055–5064.
PMID:29561018
-
Neuhaus, W., Piontek, A., Protze, J., Eichner, M., Mahringer, A., Subileau, E.-A., Lee, I.-F.M., Schulzke, J.D., Krause, G., Piontek, J., 2018. Biomaterials 161:129–143.
PMID:29421550
-
The involvement of NK1 and Y2 receptor in the development of laser-induced CNVs in C57Bl/6N mice.
Nowosielski, Y., Haas, G., Seifarth, C., Wohlfarter, W., Tasan, R., Verius, M., Troger, J., Bechrakis, N., 2018. Exp. Eye Res. 177:87–95.
PMID:30076797
-
Obermoser, V., Baecker, D., Schuster, C., Braun, V., Kircher, B., Gust, R., 2018. Dalton Transactions 47(12):4341–4351.
PMID:29492489
-
Rath, B., Hochmair, M., Plangger, A., Hamilton, G., 2018. Mar Drugs 16(10):383.
PMID:30322180; PMCID: PMC6213142
-
Antiprotozoal and cysteine proteases inhibitory activity of dipeptidyl enoates.
Royo, S., Schirmeister, T., Kaiser, M., Jung, S., Rodríguez, S., Bautista, J.M., González, F.V., 2018. Bioorg. Med. Chem. 26(16):4624–4634.
PMID:30037754
-
Schelch, K., Kirschner, M.B., Williams, M., Cheng, Y.Y., van Zandwijk, N., Grusch, M., Reid, G., 2018. Mol Oncol 12(1):58–73.
PMID:29094504; PMCID: PMC5748487
-
TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma.
Spiegl-Kreinecker, S., Lötsch, D., Neumayer, K., Kastler, L., Gojo, J., Pirker, C., Pichler, J., Weis, S., Kumar, R., Webersinke, G., Gruber, A., Berger, W., 2018. Neuro-Oncology 12;20(12):1584-1593.
PMID:30010853
-
Syring, I., Weiten, R., Müller, T., Schmidt, D., Steiner, S., Kristiansen, G., Müller, S.C., Ellinger, J., 2018. Oncology Letters 16(6):3013–3021.
PMID:30127891
-
A preclinical model for studying herpes simplex virus infection.
Tajpara, P., Mildner, M., Schmidt, R., Vierhapper, M., Matiasek, J., Popow-Kraupp, T., Schuster, C., Elbe-Bürger, A., 2018. J. Invest. Dermatol. 139(3):673-682.
PMID:30414908
-
Thurner, L., Hartmann, S., Fadle, N., Kemele, M., Bock, T., Bewarder, M., Regitz, E., Neumann, F., Nimmesgern, A., von Müller, L., Pott, C., Kim, Y.-J., Bohle, R.M., Wasik, M., Schuster, S.J., Hansmann, M.-L., Preuss, K.-D., Pfreundschuh, M., 2018. Leukemia 33: 148–158.
PMID:29955130
-
Hyper N-glycosylated SAMD14 and neurabin-I as driver CNS autoantigens of PCNSL.
Thurner, L., Preuss, K.-D., Bewarder, M., Kemele, M., Fadle, N., Regitz, E., Altmeyer, S., Schormann, C., Poeschel, V., Ziepert, M., Walter, S., Roth, P., Weller, M., Szczepanowski, M., Klapper, W., Monoranu, C., Rosenwald, A., Möller, P., Hartmann, S., Hansmann, M.-L., Mackensen, A., Schäfer, H., Schorb, E., Illerhaus, G., Buslei, R., Bohle, R.M., Stilgenbauer, S., Kim, Y.-J., Pfreundschuh, M., 2018. Blood 132(26):2744-2753.
PMID:30249786
-
Venugopal, S., Kao, C., Chandna, R., Sulochana, K.N., Subramanian, V., Chen, M., Kini, R.M., Ge, R., 2018. Angiogenesis 21(3):653–665.
PMID:29691683
-
Weingartshofer, S., Bilban, M., Kastner, M.T., Hlavaty, J., Walter, I., Grunt, T.W., Singer, C.F., 2018. JCO 36:e12570–e12570.
-
Weinmann, D., Mueller, M., Walzer, S.M., Hobusch, G.M., Lass, R., Gahleitner, C., Viernstein, H., Windhager, R., Toegel, S., 2018. J. Orthop. Res. 36(9):2431–2438.
PMID:29704279
-
Weiten, R., Müller, T., Schmidt, D., Steiner, S., Kristiansen, G., Müller, S.C., Ellinger, J., Syring, I., 2018. Cancer Biomark 21(4):839–847.
PMID:29400661
-
Załuski, M., Stanuch, K., Karcz, T., Hinz, S., Latacz, G., Szymańska, E., Schabikowski, J., Doroż-Płonka, A., Handzlik, J., Drabczyńska, A., Müller, C.E., Kieć-Kononowicz, K., 2018. MedChemComm 9(6):951-962.
PMID:30108984
-
Zlabinger, K., Lukovic, D., Hemetsberger, R., Gugerell, A., Winkler, J., Mandic, L., Traxler, D., Spannbauer, A., Wolbank, S., Zanoni, G., Kaun, C., Posa, A., Gyenes, A., Petrasi, Z., Petnehazy, Ö., Repa, I., Hofer-Warbinek, R., de Martin, R., Gruber, F., Charwat, S., Huber, K., Pavo, N., Pavo, I.J., Nyolczas, N., Kraitchman, D.L., Gyöngyösi, M., 2018. Front Bioeng Biotechnol 6:35.
PMID:29670878; PMCID: PMC5893806
-
Alenka Vesel, Nina Recek, Helena Motaln, Miran Mozetic, 2017. Plasma 1(1):12–22.
-
Englinger, B., Kallus, S., Senkiv, J., Heilos, D., Gabler, L., van Schoonhoven, S., Terenzi, A., Moser, P., Pirker, C., Timelthaler, G., Jäger, W., Kowol, C.R., Heffeter, P., Grusch, M., Berger, W., 2017. J Exp Clin Cancer Res 36(1):122.
PMID:28882160; PMCID: PMC5590147
-
Role of thiamine in Huntington’s disease pathogenesis: In vitro studies.
Gruber, B., Krzysztoń-Russjan, J., Bubko, I., Syska, J., Jaworska, M., Zmysłowski, A., Rosłon, M., Drozd, J., Drozd, E., Majorczyk, E., Anuszewska, E., 2017. Advances in Clinical and Experimental Medicine 26(5):751-760.
PMID:29068569
-
Gschwantler-Kaulich, D., Weingartshofer, S., Grunt, T.W., Mairhofer, M., Tan, Y., Gamper, J., Singer, C.F., 2017. PLOS ONE 12(9):e0185566.
PMID:28945801
-
Klameth, L., Rath, B., Hamilton, G., 2017. Journal of Cancer 8(10):1733–1743.
PMID:28819369
-
Kubista, B., Schoefl, T., Mayr, L., van Schoonhoven, S., Heffeter, P., Windhager, R., Keppler, B.K., Berger, W., 2017. Journal of Experimental & Clinical Cancer Research 36:52.
PMID:28403890
-
Mayer, B., Karakhanova, S., Bauer, N., Liu, L., Zhu, Y., Philippov, P.P., Werner, J., Bazhin, A.V., 2017. Naunyn-Schmiedeberg’s Arch Pharmacol 390(11):1125–1134.
PMID:28779210
-
Panchuk, R.R., Lehka, L.V., Terenzi, A., Matselyukh, B.P., Rohr, J., Jha, A.K., Downey, T., Kril, I.J., Herbacek, I., van Schoonhoven, S., Heffeter, P., Stoika, R.S., Berger, W., 2017. Free Radical Biology and Medicine 106:134–147.
PMID:28189848
-
Neoadjuvant therapy for resectable pancreatic cancer.
Rahman, S.H., Urquhart, R., Molinari, M., 2017. World J Gastrointest Oncol 9(12):457–465.
PMID:29290916; PMCID: PMC5740086
-
Sadek, B., Oz, M., Nurulain, S.M., Jayaprakash, P., Latacz, G., Kieć-Kononowicz, K., Szymańska, E., 2017. Epilepsy Res. 138:124–131.
PMID:28554717
-
Schoenhacker-Alte, B., Mohr, T., Pirker, C., Kryeziu, K., Kuhn, P.-S., Buck, A., Hofmann, T., Gerner, C., Hermann, G., Koellensperger, G., Keppler, B.K., Berger, W., Heffeter, P., 2017. Cancer Lett. 404:79–88.
PMID:28716523
-
The Contrasting Role of the Mediator Subunit MED30 in the Progression of Bladder Cancer.
Syring, I., Weiten, R., Müller, T., Schmidt, D., Steiner, S., Kristiansen, G., Müller, S.C., Ellinger, J., 2017. Anticancer Res. 37(12):6685–6695.
PMID:29187445
-
Platelet Lysate: The Better Choice for Jaw Periosteal Cell Mineralization.
Wanner, Y., Umrath, F., Waidmann, M., Reinert, S., Alexander, D., 2017. Stem Cells International 2017:1–10.
-
LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines.
Aschacher, T., Wolf, B., Enzmann, F., Kienzl, P., Messner, B., Sampl, S., Svoboda, M., Mechtcheriakova, D., Holzmann, K., Bergmann, M., 2016. Oncogene 35, 94–104.
PMID:25798839
-
Toxicity of Nanoparticles and an Overview of Current Experimental Models.
Bahadar, H., Maqbool, F., Niaz, K., Abdollahi, M., 2016. Iran Biomed J 20, 1–11.
PMID:26286636; PMCID: PMC4689276
-
Drozd, E., Gruber, B., Marczewska, J., Drozd, J., Anuszewska, E., 2016. Postępy Higieny i Medycyny Doświadczalnej 70, 319–328.
-
Hamilton, G., Rath, B., Holzer, S., Hochmair, M., 2016. Transl Lung Cancer Res 5, 71–77.
PMID:26958494; PMCID: PMC4758977
-
Hoda, M.A., Pirker, C., Dong, Y., Schelch, K., Heffeter, P., Kryeziu, K., van Schoonhoven, S., Klikovits, T., Laszlo, V., Rozsas, A., Ozsvar, J., Klepetko, W., Döme, B., Grusch, M., Hegedüs, B., Berger, W., 2016. Mol. Cancer Ther. 15, 2357–2369.
PMID:27512118
-
Latacz, G., Kechagioglou, P., Papi, R., Łażewska, D., Więcek, M., Kamińska, K., Wencel, P., Karcz, T., Schwed, J.S., Stark, H., Kyriakidis, D.A., Kieć-Kononowicz, K., 2016. Chem Biol Drug Des 88, 254–263.
PMID:26931395
-
Lehmann, Ricarda, Gallert, C., Roddelkopf, T., Junginger, S., Jonitz‐Heincke, A., Wree, A., Thurow, K., 2016. Engineering in Life Sciences 16, 272–282.
-
Lehmann, R., Gallert, C., Roddelkopf, T., Junginger, S., Wree, A., Thurow, K., 2016. Cytotechnology 68, 1049–1062.
PMID:25842191; PMCID: PMC4960154
-
Nandi, S., Sykes, P.D., Hasan, E., Rubbi, C., Barrow, M., Neoptolemos, J.P., Costello, E., Rosseinsky, M., Halloran, C.M., 2016. HPB 18, e776.
-
Panchuk, R.R., Lehka, L.V., Rohr, J., Berger, W., Stoika, R.S., 2016. Biopolymers & Cell 32, 190–202.
-
Effects of inorganic phosphate and FGF23 on C2C12 myoblast cells.
Raimann, A., Dangl, A., Javanmardi, A., Ertl, A., Egerbacher, M., Greber-Platzer, S., Haeusler, G., 2016. Presented at the 43rd Annual European Calcified Tissue Society Congress, BioScientifica.
-
Syring, I., Klümper, N., Offermann, A., Braun, M., Deng, M., Boehm, D., Queisser, A., von Mässenhausen, A., Brägelmann, J., Vogel, W., Schmidt, D., Majores, M., Schindler, A., Kristiansen, G., Müller, S.C., Ellinger, J., Shaikhibrahim, Z., Perner, S., 2016. Oncotarget 7, 23043–23055.
PMID:27050271; PMCID: PMC5029609
-
Szöőr, Á., Ujlaky-Nagy, L., Tóth, G., Szöllősi, J., Vereb, G., 2016. Cell. Signal. 28, 81–93.
PMID:26631574
-
Thaler, R., Maurizi, A., Roschger, P., Sturmlechner, I., Khani, F., Spitzer, S., Rumpler, M., Zwerina, J., Karlic, H., Dudakovic, A., Klaushofer, K., Teti, A., Rucci, N., Varga, F., Wijnen, A.J. van, 2016. J. Biol. Chem. jbc.M115.678235.
PMID:26757819
-
Ardjomandi, N., Henrich, A., Huth, J., Klein, C., Schweizer, E., Scheideler, L., Rupp, F., Reinert, S., Alexander, D., 2015. Biomed Mater 10, 045018.
PMID:26238604
-
Identification of novel long non-coding RNAs in clear cell renal cell carcinoma.
Blondeau, J.J., Deng, M., Syring, I., Schrödter, S., Schmidt, D., Perner, S., Müller, S.C., Ellinger, J., 2015. Clin Epigenetics 7, 10.
PMID:25685243; PMCID: PMC4326488
-
Bojar, W., Ciach, T., Kucharska, M., Maurin, J., Gruber, B., Krzysztoń-Russjan, J., Bubko, I., Anuszewska, E., 2015. Advances in Clinical and Experimental Medicine 24, 511–516.
-
Developmental Regulation and Induction of Cytochrome P450 2W1, an Enzyme Expressed in Colon Tumors.
Choong, E., Guo, J., Persson, A., Virding, S., Johansson, I., Mkrtchian, S., Ingelman-Sundberg, M., 2015. PLOS ONE 10, e0122820.
-
Duran Lengua, M., Kamali, A.N., Cano, A.J., Piermattey, J., Reyes, N., Bautista, J.M., Gaitan, R., 2015. African Journal of Pharmacy and Pharmacology 9, 595–602.
-
Effects of non-toxic zinc exposure on human epidermal keratinocytes.
Emri, E., Miko, E., Bai, P., Boros, G., Nagy, G., Rózsa, D., Juhász, T., Hegedűs, C., Horkay, I., Remenyik, É., Emri, G., 2015. Metallomics 7, 499–507.
-
Biological high throughput screening of 2D and 3D cell cultures for future industrial up-scaling.
Gallert, C., Lehmann, R., Roddelkopf, T., Junginger, S., Thurow, K., 2015, in: 2015 IEEE International Conference on Automation Science and Engineering (CASE). Presented at the 2015 IEEE International Conference on Automation Science and Engineering (CASE), pp. 1527–1532.
-
Activin A is anti-lymphangiogenic in a melanoma mouse model.
Heinz, M., Niederleithner, H.L., Puujalka, E., Soler-Cardona, A., Grusch, M., Pehamberger, H., Loewe, R., Petzelbauer, P., 2015. J. Invest. Dermatol. 135, 212–221.
PMID:25084052
-
Miklos, W., Pelivan, K., Kowol, C.R., Pirker, C., Dornetshuber-Fleiss, R., Spitzwieser, M., Englinger, B., van Schoonhoven, S., Cichna-Markl, M., Koellensperger, G., Keppler, B.K., Berger, W., Heffeter, P., 2015. Cancer Lett. 361, 112–120.
PMID:25749419
-
Balanced Hydroxyethylstarch (HES 130/0.4) Impairs Kidney Function In-Vivo without Inflammation.
Schick, M.A., Baar, W., Bruno, R.R., Wollborn, J., Held, C., Schneider, R., Flemming, S., Schlegel, N., Roewer, N., Neuhaus, W., Wunder, C., 2015. PLoS ONE 10, e0137247.
PMID:26340751; PMCID: PMC4560431
-
Sevelda, F., Mayr, L., Kubista, B., Lötsch, D., van Schoonhoven, S., Windhager, R., Pirker, C., Micksche, M., Berger, W., 2015. Journal of Experimental & Clinical Cancer Research 34, 134.
-
The molecular basis for development of proinflammatory autoantibodies to progranulin.
Thurner, L., Fadle, N., Regitz, E., Kemele, M., Klemm, P., Zaks, M., Stöger, E., Bette, B., Carbon, G., Zimmer, V., Assmann, G., Murawski, N., Kubuschok, B., Held, G., Preuss, K.-D., Pfreundschuh, M., 2015. J. Autoimmun. 61, 17–28.
PMID:26005049
-
Wollborn, J., Wunder, C., Stix, J., Neuhaus, W., Bruno, R.R., Baar, W., Flemming, S., Roewer, N., Schlegel, N., Schick, M.A., 2015. J Pharmacol Pharmacother 6, 13–23.
PMID:25709347; PMCID: PMC4319242
-
Zygmunt, M., Sapa, J., Drabczyńska, A., Karcz, T., Müller, C., Köse, M., Latacz, G., Schabikowski, J., Bednarski, M., Kieć-Kononowicz, K., 2015. Arch. Pharm. 348, 704–714.
PMID:26248713
-
In toto differentiation of human amniotic membrane towards the Schwann cell lineage.
Banerjee, A., Nürnberger, S., Hennerbichler, S., Riedl, S., Schuh, C.M.A.P., Hacobian, A., Teuschl, A., Eibl, J., Redl, H., Wolbank, S., 2014. Cell Tissue Bank 15, 227–239.
PMID:24166477
-
Quinoid Compounds Cause Inhibition of Falcipain 2, and Arrest Plasmodium falciparum Growth in Vitro.
DURAN-LENGUA, M., SALAS-SARDUY, E., CANO-DURAN, L.M., MONERIZ-PRETELL, C.E., MÉNDEZ-CUADRO, D.M., LORENZO-RIVERA, J., PIERMATTEY-DITTA, J., MONTALVO-ACOSTA, J., CRUZ, J.M.B.-S., IBARRA, R.G., 2014. Latin American Journal of Pharmacy 9
-
The study of cellular cytotoxicity of argireline - an anti-aging peptide.
Grosicki, M., Latacz, G., Szopa, A., Cukier, A., Kieć-Kononowicz, K., 2014. Acta Biochimica Polonica 61
-
Hamilton, G., Klameth, L., Rath, B., Thalhammer, T., 2014. Molecules 19, 2077–2088.
PMID:24549232; PMCID: PMC6271949
-
Long-term chronic toxicity testing using human pluripotent stem cell-derived hepatocytes.
Holmgren, G., Sjögren, A.-K., Barragan, I., Sabirsh, A., Sartipy, P., Synnergren, J., Björquist, P., Ingelman-Sundberg, M., Andersson, T.B., Edsbagge, J., 2014. Drug Metab. Dispos. 42, 1401–1406.
PMID:24980256
-
Combination therapy using a novel Plk-1 inhibitor and gemcitabine in pancreatic cancer cells.
Jones, O., Greenhalf, W. (Bill), Halloran, C., Ghaneh, P., 2014. Pancreatology 14, S21.
PMID:24555975
-
Laurent, R., Nallet, A., Obert, L., Nicod, L., Gindraux, F., 2014. Cell Tissue Bank 15, 267–275.
PMID:24633398
-
Intact human amniotic membrane differentiated towards the chondrogenic lineage.
Lindenmair, A., Nürnberger, S., Stadler, G., Meinl, A., Hackl, C., Eibl, J., Gabriel, C., Hennerbichler, S., Redl, H., Wolbank, S., 2014. Cell Tissue Bank 15, 213–225.
PMID:24828570
-
Autophagy mediates resistance to gemcitabine treatment through a novel E2F1-p300-VMP1 pathway.
Vaccaro, M.I., Ropolo, A., Molejon, M.I., Catrinacio, C., Renna, F., Boggio, V., Gonzalez, C., 2014. Pancreatology 14, S21.
PMID:24555975
-
Zhu, Y., Karakhanova, S., Huang, X., Deng, S. ping, Werner, J., Bazhin, A.V., 2014. Experimental Cell Research 324, 146–156.
-
Kryeziu, K., Jungwirth, U., Hoda, M.A., Ferk, F., Knasmüller, S., Karnthaler-Benbakka, C., Kowol, C.R., Berger, W., Heffeter, P., 2013. Mol. Cancer Ther. 12, 1073–1084.
PMID: 23548265
-
Travica, S., Pors, K., Loadman, P.M., Shnyder, S.D., Johansson, I., Alandas, M.N., Sheldrake, H.M., Mkrtchian, S., Patterson, L.H., Ingelman-Sundberg, M., 2013. Clin. Cancer Res. 19, 2952–2961.
PMID:23589180
-
Zwick, C., Fadle, N., Regitz, E., Kemele, M., Stilgenbauer, S., Bühler, A., Pfreundschuh, M., Preuss, K.-D., 2013. Blood 121, 4708–4717.
PMID:23580660
-
Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis.
Cao, R., Ji, H., Feng, N., Zhang, Y., Yang, X., Andersson, P., Sun, Y., Tritsaris, K., Hansen, A.J., Dissing, S., Cao, Y., 2012. Proc. Natl. Acad. Sci. U.S.A. 109, 15894–15899.
PMID:22967508; PMCID: PMC3465417
-
Anticancer activity of methyl-substituted oxaliplatin analogs.
Jungwirth, U., Xanthos, D.N., Gojo, J., Bytzek, A.K., Körner, W., Heffeter, P., Abramkin, S.A., Jakupec, M.A., Hartinger, C.G., Windberger, U., Galanski, M., Keppler, B.K., Berger, W., 2012. Mol. Pharmacol. 81, 719–728.
PMID:22331606; PMCID: PMC3375001
-
MET expression in melanoma correlates with a lymphangiogenic phenotype.
Swoboda, A., Schanab, O., Tauber, S., Bilban, M., Berger, W., Petzelbauer, P., Mikula, M., 2012. Hum. Mol. Genet. 21, 3387–3396.
PMID:22570180
-
Breinig, M., Mayer, P., Harjung, A., Goeppert, B., Malz, M., Penzel, R., Neumann, O., Hartmann, A., Dienemann, H., Giaccone, G., Schirmacher, P., Kern, M.A., Chiosis, G., Rieker, R.J., 2011. Clin. Cancer Res. 17, 2237–2249.
PMID:21372220
-
Ang, C.W., Nedjadi, T., Sheikh, A.A., Tweedle, E.M., Tonack, S., Honap, S., Jenkins, R.E., Park, B.K., Schwarte-Waldhoff, I., Khattak, I., Azadeh, B., Dodson, A., Kalirai, H., Neoptolemos, J.P., Rooney, P.S., Costello, E., 2010. Carcinogenesis 31, 1541–1551.
PMID:20622003
-
Cindric, M., Cipak, A., Serly, J., Plotniece, A., Jaganjac, M., Mrakovcic, L., Lovakovic, T., Dedic, A., Soldo, I., Duburs, G., Zarkovic, N., Molnár, J., 2010. Anticancer Res. 30, 4063–4069.
PMID:21036720
-
Gomez, A., Nekvindova, J., Travica, S., Lee, M.-Y., Johansson, I., Edler, D., Mkrtchian, S., Ingelman-Sundberg, M., 2010. Mol. Pharmacol. 78, 1004–1011.
PMID:20805301
-
Shehata, M., Schnabl, S., Demirtas, D., Hilgarth, M., Hubmann, R., Ponath, E., Badrnya, S., Lehner, C., Hoelbl, A., Duechler, M., Gaiger, A., Zielinski, C., Schwarzmeier, J.D., Jaeger, U., 2010. Blood 116, 2513–2521.
PMID:20576813
-
The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls.
Vercauteren, D., Vandenbroucke, R.E., Jones, A.T., Rejman, J., Demeester, J., De Smedt, S.C., Sanders, N.N., Braeckmans, K., 2010. Mol. Ther. 18, 561–569.
PMID:20010917; PMCID: PMC2839427
-
Hoberg, M., Schmidt, E.L., Tuerk, M., Stark, V., Aicher, W.K., Rudert, M., 2009. Archives of Orthopaedic and Trauma Surgery 129, 1137–1143.
-
NF-kappaB independent activation of a series of proinflammatory genes by hydrogen sulfide.
Stuhlmeier, K.M., Bröll, J., Iliev, B., 2009. Exp. Biol. Med. 234, 1327–1338.
PMID:19855074
-
Anti-acanthamoeba efficacy and toxicity of miltefosine in an organotypic skin equivalent.
Walochnik, J., Obwaller, A., Gruber, F., Mildner, M., Tschachler, E., Suchomel, M., Duchêne, M., Auer, H., 2009. J. Antimicrob. Chemother. 64, 539–545.
PMID:19549672
-
Differential effects of rapamycin in anti-GBM glomerulonephritis.
Hochegger, K., Jansky, G.L., Soleiman, A., Wolf, A.M., Tagwerker, A., Seger, C., Griesmacher, A., Mayer, G., Rosenkranz, A.R., 2008. J. Am. Soc. Nephrol. 19, 1520–1529.
PMID:18480312; PMCID: PMC2488269
-
FGF18 in colorectal tumour cells: autocrine and paracrine effects.
Sonvilla, G., Allerstorfer, S., Stättner, S., Karner, J., Klimpfinger, M., Fischer, H., Grasl-Kraupp, B., Holzmann, K., Berger, W., Wrba, F., Marian, B., Grusch, M., 2008. Carcinogenesis 29, 15–24.
PMID:17890768
-
Demyanets, S., Kaun, C., Rychli, K., Rega, G., Pfaffenberger, S., Afonyushkin, T., Bochkov, V.N., Maurer, G., Huber, K., Wojta, J., 2007. Am. J. Physiol. Heart Circ. Physiol. 293, H1962-1968.
PMID:17604327
-
Emanuele, S., Lauricella, M., Carlisi, D., Vassallo, B., D’Anneo, A., Di Fazio, P., Vento, R., Tesoriere, G., 2007. Apoptosis 12, 1327–1338.
PMID:17351739
-
Molnarfi, N., Gruaz, L., Dayer, J.-M., Burger, D., 2007. J. Immunol. 178(1):446–454.
PMID:17182583
-
Tonack, S., Kind, K., Thompson, J.G., Wobus, A.M., Fischer, B., Santos, A.N., 2007. Endocrinology 148(12):5902–5912.
PMID:17872374
-
Structure and in vitro cytocompatibility of the gastropod shell of Helix pomatia.
Bächle, M., Hübner, U., Kohal, R.J., Han, J.S., Wiedmann-Al-Ahmad, M., 2006. Tissue Cell 38(5):337–344.
PMID:17010402
-
Structure-activity profiles of Ab-derived TNF fusion proteins.
Bauer, S., Adrian, N., Fischer, E., Kleber, S., Stenner, F., Wadle, A., Fadle, N., Zoellner, A., Bernhardt, R., Knuth, A., Old, L.J., Renner, C., 2006. J. Immunol. 177(4):2423–2430.
PMID:16888004
-
Fineschi, S., Reith, W., Guerne, P.A., Dayer, J.-M., Chizzolini, C., 2006. FASEB J. 20(3):562–564.
PMID:16410344
-
Gruszka, A., Kunert-Radek, J., Radek, A., Pisarek, H., Taylor, J., Dong, J.Z., Culler, M.D., Pawlikowski, M., 2006. Life Sci. 78(7):689–693.
PMID:16115652
-
Influence of Cyclosporin A on human gingival keratinocytes in vitro.
Lauer, G., Mai, R., Pradel, W., Proff, P., Gedrange, T., Beyer, J., 2006. J Craniomaxillofac Surg 34(Suppl 2):116–122.
PMID:17071404
-
Meerbach, A., Meier, C., Sauerbrei, A., Meckel, H.-M., Wutzler, P., 2006. Int. J. Antimicrob. Agents 27(5):423–430.
PMID:16621459
-
Perabo, F.G.E., Frössler, C., Landwehrs, G., Schmidt, D.H., von Rücker, A., Wirger, A., Müller, S.C., 2006. Anticancer Res. 26(3A):2129–2135.
PMID:16827155
-
Tepel, J., Dagvadorj, O., Kapischke, M., Sipos, B., Leins, A., Kremer, B., Kalthoff, H., 2006. Int J Colorectal Dis 21(4):365–372.
PMID:16133009
-
Yoshida, A., Kohchi, C., Inagawa, H., Nishizawa, T., Hori, H., Soma, G.-I., 2006. Anticancer Res. 26(6A):4003–4007.
PMID:17195449
-
Age-dependent role for CCR5 in antiviral host defense against herpes simplex virus type 2.
Ank, N., Petersen, K., Malmgaard, L., Mogensen, S.C., Paludan, S.R., 2005. J. Virol. 79(15):9831–9841.
PMID:16014944; PMCID: PMC1181601
-
Heffeter, P., Pongratz, M., Steiner, E., Chiba, P., Jakupec, M.A., Elbling, L., Marian, B., Körner, W., Sevelda, F., Micksche, M., Keppler, B.K., Berger, W., 2005. J. Pharmacol. Exp. Ther. 312(1):281–289.
PMID:15331656
-
Biological Characterization of Novel Inhibitors of the Gram-Positive DNA Polymerase IIIC Enzyme.
Kuhl, A., Svenstrup, N., Ladel, C., Otteneder, M., Binas, A., Schiffer, G., Brands, M., Lampe, T., Ziegelbauer, K., Rübsamen-Waigmann, H., Haebich, D., Ehlert, K., 2005. Antimicrob. Agents Chemother. 49(3):987–995.
PMID:15728893
-
Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis.
Sulochana, K.N., Fan, H., Jois, S., Subramanian, V., Sun, F., Kini, R.M., Ge, R., 2005. J. Biol. Chem. 280:27935–27948.
PMID:15923192
-
Wegner, B., Baer, P., Gauer, S., Oremek, G., Hauser, I.A., Geiger, H., 2005. Nephrol. Dial. Transplant. 20(10):2071–2079.
PMID:15998654
-
Targeted bioactivity of membrane-anchored TNF by an antibody-derived TNF fusion protein.
Bauer, S., Adrian, N., Williamson, B., Panousis, C., Fadle, N., Smerd, J., Fettah, I., Scott, A.M., Pfreundschuh, M., Renner, C., 2004. J. Immunol. 172(6):3930–3939
PMID:15004201
-
Thurow, K., Entzian, K., Eberlein, G., 2004. JALA: Journal of the Association for Laboratory Automation 9(3):159–162.
-
Verhulst, A., D’Haese, P.C., De Broe, M.E., 2004. J. Am. Soc. Nephrol. 15(9):2249–2257.
PMID:15339974
-
Völlenkle, C., Weigert, S., Ilk, N., Egelseer, E., Weber, V., Loth, F., Falkenhagen, D., Sleytr, U.B., Sára, M., 2004. Appl. Environ. Microbiol. 70(3):1514–1521
PMID:15006773; PMCID: PMC368406
-
Hutter, R., Sauter, B.V., Reis, E.D., Roque, M., Vorchheimer, D., Carrick, F.E., Fallon, J.T., Fuster, V., Badimon, J.J., 2003. Circulation 107:1658–1663.
PMID:12668502
-
Sturlan, S., Baumgartner, M., Roth, E., Bachleitner-Hofmann, T., 2003. Blood 101(12):4990–4997.
PMID:12609832
-
Oxytocin stimulates proliferation of human osteoblast-like cells.
Petersson, M., Lagumdzija, A., Stark, A., Bucht, E., 2002. Peptides 23(6):1121–1126.
PMID:12126740
-
Lin, Y., Kreeft, A., Schuurbiers, J.A.E., Draijer, R., 2001. The Journal of Nutritional Biochemistry 12(3):183–189.
PMID:11257467
-
In vitro toxicity of surfactants in U937 cells: Cell membrane integrity and mitochondrial function.
Jelinek, A., Klöcking, H.-P., 1998. Experimental and Toxicologic Pathology 50(4-6):472–476.
PMID:9784025
-
Measuring Cell Proliferation with EZ4U
We wanted to check for the proliferation of murine T cells upon activation with anti CD3/CD28. This kit gave good result to indicate proliferation of activated T cells.
Application: Proliferation assay
Starting Material: Murine pan T cells
Results Summary: The result showed proliferation of activated T cells
The Good
Easy and fastThe Bad
A bit difficult to reproduce the resultThe Bottom Line
Easy to use kit
 Download biomedica product list
Download biomedica product list