Borrelia IgM ELISA | BI-21042
-
Method
Indirect ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
Serum, plasma and CSF
-
Sample volume
10 µl / sample (serum and plasma), 100 µl / sample (CSF)
-
Assay time
1 h / 30 min / 15 min
-
Standard range
0 – 30 BBU/ml (Biomedica Borrelia Units)
-
Sensitivity
100% (serum)
82% (CSF)
-
Cross-reactivity
The assay works with human samples only.
-
Specificity
Endogenous and recombinant human anti-Borrelia IgM (B. burgdoreri ss, B. afzelii, B. garnii, B. bavariensis)
-
Use
CE marked – for IVD use in the EU
-
Validation Data
See validation data tab for: precision, specificity, sensitivity
Borrelia IgM ELISA Product Overview
The Borrelia assay is a 2 hour, 96-well indirect ELISA for the qualitative or quantitative determination of Borrelia IgM antibodies in human serum, plasma and CSF samples. The assay employs human serum-based standards to ensure the measurement of biologically reliable data.
The Borrelia test kit uses a recombinant antigen mix to improve the diagnostic specificity:
- p21 = OspC (outer surface protein C): B. afzellii (pKo), B. garinii (20047)
- p41/I = (inner part of flagellin): B. bavariensis (pBi)
- VIsE: fusion proteins of different Borrelia genospecies
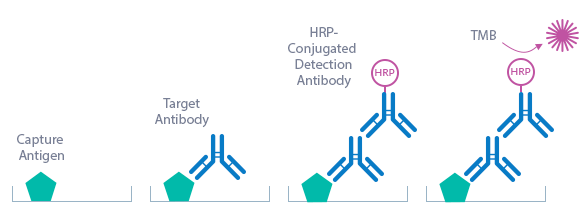
Borrelia IgM ELISA Assay Principle
The Borrelia IgM ELISA kit is an indirect enzyme immunoassay for the qualitative or quantitative determination of Borrelia IgM antibodies in human serum, plasma and CSF samples.
The figure below explains the principle of the Borrelia ELISA:
Capture antigen: recombinant Borrelia antigens (see Product Overview)
Detection antibody: HRP-conjugated rabbit anti-human IgM antibody
Target: human anti-Borrelia IgM antibodies
In a first step, the standard/sample (STD/SAMPLE) is pipetted into the wells of the microtiter strips. IgM antibodies present in the STD/SAMPLE bind to the antigen mix that is pre-coated in the wells. After incubation, the plate is washed. This removes all non-specific unbound material. In a next step, the conjugate (HRP-conjugated rabbit anti-human IgM antibody) is pipetted into the wells and reacts with the anti-Borrelia IgM antibodies present in the sample. After another washing step, the substrate (Tetramethylbenzidine; TMB) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is directly proportional to the amount of IgG antibody present in the sample. This color change is detectable with a standard microtiter plate reader. The optical density (OD) of the cut-off STD represents 10 BBU/ml (Biomedica Borrelia units). This is the basis for the calculation of the results; see calculation of results in the instructions for use section for more information.
Borrelia IgM ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Detachable microtiter strips, breakable, color coded, coated with recombinant antigen |
12 x 8 tests |
|
STD |
Human serum-based standards: positive (pos), cut-off (c.o.), negative (neg), ready to use |
Each 1 x 1.5 ml |
|
CONJ |
Conjuaget, HRP-conjugated rabbit anti-human IgM antibodies |
1 x 14 ml |
|
DIL |
Sample diluent with green dye, ready to use |
1 x 105 ml |
|
SUB |
Tetramethylbenzidine (TMB) substrate solution, ready to use |
1 x 13 ml |
|
WASHBUF |
Wash buffer: 20 x concentrate |
1 x 50 ml |
|
STOP |
Stop solution: sulfuric acid, ready to use |
1 x 10 ml |
Storage instructions: All reagents of the Borrelia IgM ELISA kit as supplied are stable at 4°C until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Serum, EDTA plasma, heparın plasma, citrate plasma, CPD plasma and CSF are suitable for use in this assay. Do not change sample type during studies. We recommend duplicate measurements for all samples and standards. The sample collection and storage conditions listed are intended as general guidelines.
Serum & Plasma
Collect venous blood samples in standardized serum separator tubes (SST) or standardized blood collection tubes using EDTA, heparın, citrate or CPD as an anticoagulant. For serum samples, allow samples to clot for 30 minutes at room temperature. Perform separation by centrifugation according to the tube manufacturer’s instructions for use. The use of heat-inactivated, icteric, haemolytic, lipemic or turbid samples is not recommended. If the tests are not carried out immediately, the samples can be stored for up to two weeks at 2-8°C. Prolonged storage of the samples is possible at -25°C or below. Avoid more than three freeze-thaw cycles.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50ml WASHBUF + 950ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Standards for Serum and Plasma
Standards are ready-to-use and MUST NOT be diluted.
Sample Preparation
Sample Dilution
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
Serum/plasma: dilute sample 1:101 (1 + 100) with sample diluent, e.g. 10 µl of sample + 1 ml of sample diluent, mix well.
CSF: dilute cerebrospinal fluid 1:2 (1 + 1) with sample diluent, e.g. 100 µl of sample + 100 µl of sample diluent, mix well.
For samples with results >30 BBU (Biomedica Borrelia Units)/ml the samples can be diluted with sample dilution buffer and retested. The obtained results need to be multiplied with their respective dilution factor to determine the final concentration.
Borrelia IgM ELISA Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring samples and reagents to room temperature (18-26°C). |
|
2. |
Mark positions for BLANK/STD/SAMPLE (blank/standard/sample) on the protocol sheet. Take a minimum of one well as blank. |
|
3. |
Take the microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
4. |
Pipette 100 µl STD/SAMPLE (standards, diluted sample) into the respective wells. Leave wells for blank measurement empty. |
|
5. |
Cover strips with the plastic film and incubate for 1 hour at 37°C in an incubator. |
|
6. |
Aspirate and wash wells 4 x with at least 250 µl diluted WASHBUF (wash buffer). Remove remaining WASHBUF by tapping the plate against paper towel after the last wash. |
|
7. |
Add 100 µl CONJ (HRP-conjugated anti-human IgM) into each well. |
|
8. |
Cover strips with plastic film and incubate for 30 min at room temperature (18-26°C). |
|
9. |
Aspirate and wash wells 4 x with at least 250 µl diluted WASHBUF (wash buffer). Remove remaining WASHBUF by tapping the plate against paper towel after the last wash. |
|
10. |
Add 100 µl SUB (TMB substrate) into each well. |
|
11. |
Incubate 15 min at room temperature (18-26°C) in the dark. |
|
12. |
Add 50 µl STOP (stop solution) into each well. Swirl gently. |
|
13. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Subtract the optical density (OD) of the blank from the OD’s of the samples and standards.
Calculate the mean OD’s of all samples and standards.
The OD of the cutoff standard represents 10 BBU/ml (Biomedica Borrelia Units). This is the basis for calculation of the results.
The quality control protocol supplied with the kit shows the results of the final release QC for each kit. OD data obtained by customers may differ due to various influences and/or due to the normal decrease of signal intensity during shelf life. However, this does not affect validity of results as long as the following results are achieved:
|
Blank |
The OD must be ≤ 0.250. |
|
Negative standard |
The OD must be ≤ 0.200 (blank subtracted). |
|
Cut-off standard |
The OD must be at least twice the negative standard (blank subtracted). |
|
Positive standard |
The OD must be at least twice the OD of the cut-off standard (blank subtracted). |
Qualitative Calculation for Serum/Plasma
|
Positive result |
Samples with an OD of more than cut-off +10% (≥ 11 BBU/ml) |
|
Borderline result |
Samples between 9 - 11 BBU/ml. These patients should be retested with a new sample. |
|
Negative result |
Samples with an OD of less than cut-off -10% (≤ 9 BBU/ml) |
Quantitative Calculation for Serum/Plasma
Calculation of the BBU/ml is identical to the qualitative calculation.
The test is linear between 5 - 30 BBU/ml. Values outside this range are semi-quantitative.
Alternatively, samples with results >30 BBU/ml can be diluted with sample dilution buffer and retested. Obtained results need to be multiplied with respective dilution factor for final concentration.
|
Weak positive |
Samples between 11 - 20 BBU/ml |
|
High positive |
Samples between 21 - 30 BBU/ml |
|
Very high positive |
Samples above 30 BBU/ml |
Qualitative Calculation for Cerebrospinal Fluid
The calculation of the BBU/ml is identical to the qualitative calculation.
|
Normal values CSF |
The mean of 30 CSF samples was 1.3 BBU/ml. |
|
Neuroborreliosis negative |
Samples with values ≤ 5 BBU/ml |
|
Suspected for neuroborreliosis |
Samples with values > 5 BBU/ml |
Lyme borreliosis is a bacterial infection caused by the spirochete Borrelia Burgdorferi, Borrelia Afzelii and Borrelia Garinii and is the most vector-transmitted human disease in Europe. The spirochetal bacteria were first described and isolated in 1981 by Willi Burgdorfer in the tick Ixodes dammini in Montana (USA). The infection with Borrelia is characterized by a variety of clinical symptoms and can be divided into 3 stages:
- Stage 1, early dermatitis, appearing days or weeks after the infection. Clinical: erythema migrans.
- Stage 2, early disseminated infection, appears weeks to months after infection. Clinical: lymphocytic meningoradiculitis (Bannwarth's syndrome), neuroborreliosis.
- Stage 3, late disseminated infection, occurring up to years after infection. Clinical: chronic progressive encephalomyelitis, acrodermatitis chronica atrophicans (ACA), chronic arthritis.
Antibiotic therapy is indicated in all stages of the disease. It shortens disease duration significantly, when instituted in stage 1. Diagnosis of the bacterial infection relies on the detection of specific IgM antibodies against Borrelia.
To ensure diagnostic specificity, the Borrelia IgM ELISA microwell strips are coated with the following Borellia-specific recombinant antigens:
-
p21 = OspC (outer surface protein C): B. afzellii (pKo), B. garinii (20047)
-
p41/I = (inner part of flagellin): B. bavariensis (pBi)
-
VIsE: fusion proteins of different Borrelia genospecies
Many features of later infection are not specific to Lyme Borreliosis and occur in other conditions. The diagnosis of Lyme Borreliosis should be made only after careful evaluation of the patient´s clinical history, physical findings, laboratory evidence and exposure risk evaluation.
Borrelia IgM ELISA Sensitivity & Specificity
|
Borrelia IgG |
||
|
Serum |
CSF |
|
|
Sensitivity |
100% |
91% |
|
Specificity |
96% |
82% |
The data for specificity and sensitivity are internal data from Biomedica. They were received from following serum panel:
|
|
Clinical diagnosis |
Number of samples |
|
Lipaemic sera |
No Borreliosis |
9 sera |
|
Haemolytic sera |
No Borreliosis |
12 sera |
|
Early infection state |
Tick bite with Erythema Migrans |
5 sera |
|
Later stage infection |
Clinically defined positive sera |
30 sera |
The results were confirmed by Western Blot analysis using the product MG-4203, Borrelia IgM Western Blot from MIKROGEN GmbH, Martinsried, Germany.
Borrelia IgM ELISA Crossreactivity
| n | Crossreactive | |
| Rheumafactor-positive | 15 | 1 |
| Syphilis-positive | 7 | 0 |
This ELISA uses recombinant antigens. Nevertheless, crossreactions to autoimmune-positive samples cannot be completely excluded.
Borrelia IgM ELISA Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision
Within-run precision was tested by measuring the same samples eight times within Borrelia IgG ELISA lot.
|
ID |
n |
Mean (BBU/ml) |
SD (BBU/ml) |
CV (%) |
|
Positive |
12 |
24 |
2 |
6.7 |
|
High positive |
12 |
38 |
3 |
6.8 |
In-Between-Run Precision
In-between-run precision was assessed by measuring the same samples twelve times within multiple Borrelia IgG ELISA lots.
|
ID |
n |
Mean (BBU/ml) |
SD (BBU/ml) |
CV (%) |
|
Positive |
17 |
22 |
2 |
8.3 |
|
High positive |
20 |
36 |
3 |
8.1 |
Comparison with other Assays
A total of 32 samples has been tested with the Borrelia ELISA and a very good correlation to the results with the ELISA kits from Behring, Mikrogen and Virotech was found (anonymized Competitor ELISA A-D). The ELISA results were confirmed by Western Blots.
|
|
Competitor ELISA |
Western Blot |
|||||
|
|
Biomedica |
A |
B |
C |
D |
Mikrogen |
Virotech |
|
Positive |
15 |
9 |
8 |
15 |
9 |
15 |
15 |
|
Borderline |
2 |
0 |
1 |
1 |
0 |
0 |
7 |
|
Negative |
11 |
19 |
6 |
12 |
19 |
13 |
5 |
- Assessment of Anaplasma phagocytophilum presence in early Lyme borreliosis manifested by erythema migrans skin lesions.
Moniuszko-Malinowska, A., Dunaj, J., Andersson, M.O., Czupryna, P., Zajkowska, J., Guziejko, K., Garkowski, A., Grygorczuk, S., Kondrusik, M., Pancewicz, S., 2020. Travel Medicine and Infectious Disease 101648.
-
Pawełczyk, A., Bednarska, M., Kowalska, J.D., Uszyńska-Kałuża, B., Radkowski, M., Welc-Falęciak, R., 2019. Sci Rep 9.
PMID:30765826; PMCID: PMC6376038
- Analysis of cases of Lyme arthritis in patients hospitalized in Infectious Diseases Department, University Hospital in Cracow.
Stażyk, K., Czepiel, J., Gumulska, M., Garlicki, A., Biesiada, G., 2019 59, 5–14.
-
Tick-borne infections and co-infections in patients with non-specific symptoms in Poland.
Dunaj, J., Moniuszko-Malinowska, A., Swiecicka, I., Andersson, M., Czupryna, P., Rutkowski, K., Zambrowski, G., Zajkowska, J., Grygorczuk, S., Kondrusik, M., Świerzbińska, R., Pancewicz, S., 2018. Adv Med Sci 63, 167–172.
PMID:29120859
-
Kiewra, D., Szymanowski, M., Zalewska, G., Dobracka, B., Dobracki, W., Klakočar, J., Czułowska, A., Plewa-Tutaj, K., 2018. Int J Environ Health Res 28, 502–510.
PMID:29963907
-
Plasma lipidomic profile signature of rheumatoid arthritis versus Lyme arthritis patients.
Łuczaj, W., Moniuszko-Malinowska, A., Domingues, P., Domingues, M.R., Gindzienska-Sieskiewicz, E., Skrzydlewska, E., 2018. Arch. Biochem. Biophys. 654, 105–114.
PMID:30059653
-
Acrodermatitis chronica atrophicans: various faces of the late form of Lyme borreliosis.
Moniuszko-Malinowska, A., Czupryna, P., Dunaj, J., Pancewicz, S., Garkowski, A., Kondrusik, M., Grygorczuk, S., Zajkowska, J., 2018a. Adv Dermatol Allergol 35, 490–494.
-
Assessment of HMGB-1 concentration in tick-borne encephalitis and neuroborreliosis.
Moniuszko-Malinowska, A., Penza, P., Czupryna, P., Zajkowska, O., Pancewicz, S., Świerzbińska, R., Dunaj, J., Zajkowska, J., 2018b. Int. J. Infect. Dis. 70, 131–136.
PMID:29559369
-
Welc-Falęciak, R., Kowalska, J.D., Bednarska, M., Szatan, M., Pawełczyk, A., 2018. BMC Infect. Dis. 18, 227.
PMID:29776392; PMCID: PMC5960136
-
Moniuszko-Malinowska, A., Czupryna, P., Dunaj, J., Swierzbinska, R., Guziejko, K., Rutkowski, R., Zajkowska, J., Grygorczuk, S., Kondrusik, M., Pancewicz, S., 2017. Cytokine 90, 155–160.
-
Design, construction and evaluation of multi-epitope antigens for diagnosis of Lyme disease.
Schreterova, E., Bhide, M., Potocnakova, L., Pulzova, L.B., 2017. Ann Agric Environ Med. 24, 696–701.
-
Lyme disease in Poland – A serious problem?
Czupryna, P., Moniuszko-Malinowska, A., Pancewicz, S., Garkowski, A., Gościk, J., Siemieniako, A., Zajkowska, J., 2016. Advances in Medical Sciences 61, 96–100.
-
Lipid peroxidation in the pathogenesis of neuroborreliosis.
Moniuszko-Malinowska, A., Łuczaj, W., Jarocka-Karpowicz, I., Pancewicz, S., Zajkowska, J., Andrisic, L., Zarkovic, N., Skrzydlewska, E., 2016. Free Radical Biology and Medicine 96, 255–263.
-
Moniuszko, A., Dunaj, J., Zajkowska, J., Czupryna, P., Świerzbińska, R., Guziejko, K., Aleksiejczuk, P., Barry, G., Kondrusik, M., Pancewicz, S., 2015. Postepy Dermatol Alergol 32, 11–14.
PMID:25821421; PMCID: PMC4360001
-
Moniuszko, A., Dunaj, J., Święcicka, I., Zambrowski, G., Chmielewska-Badora, J., Żukiewicz-Sobczak, W., Zajkowska, J., Czupryna, P., Kondrusik, M., Grygorczuk, S., Swierzbinska, R., Pancewicz, S., 2014. Eur J Clin Microbiol Infect Dis 33, 1835–1841.
-
J, C.-B., A, M., W, Z.-S., J, Z., J, P., S, P., 2012. Annals of Agricultural and Environmental Medicine 19
-
Lipid peroxidation products as potential bioindicators of Lyme arthritis.
Łuczaj, W., Moniuszko, A., Rusak, M., Pancewicz, S., Zajkowska, J., Skrzydlewska, E., 2011. Eur J Clin Microbiol Infect Dis 30, 415–422.
-
Miąskiewicz, K., Walczak, E., Roguska, K., Noworyta, J., Brasse-Rumin, M., Biernacka, E., Legatowicz-Koprowska, M., Palacz, A., Lewandowski, P., Ząbek, J., 2011. Reumatologia/Rheumatology 49, 328–334
-
Leschnik, M.W., Kirtz, G., Khanakah, G., Duscher, G., Leidinger, E., Thalhammer, J.G., Joachim, A., Stanek, G., 2010. Clin. Vaccine Immunol. 17, 828–835.
PMID:20219882
-
Pawinska, A., Vogtt, E., Dzierzanowska-Fangrat, K., 2010. Pediatric Research 68, 439.
-
Levels of sVCAM-1 and sICAM-1 in patients with lyme disease.
Biesiada, G., Czepiel, J., Sobczyk-Krupiarz, I., Salamon, D., Garlicki, A., Mach, T.H., 2009. Polskie Archiwum Medycyny Wewnetrznej 119, 200–204.
-
May Lyme borreliosis lead to heart transplantation?; a case report.
Maroszyńska-Dmoch, E., Wożakowska-Kapłon, B., 2009. Kardiologia Polska (Polish Heart Journal) 67, 516–520
-
Ołdak, E., Rozkiewicz, D., Sulik, A., 2008a. Przegl Epidemiol 62 Suppl 1, 77–82
PMID:22320039
-
The significance of immunoblot tests in diagnosis of Lyme borreliosis in children.
Ołdak, E., Sulik, A., Rozkiewicz, D., 2008. Przegl Epidemiol 62 Suppl 1, 83–87
PMID:22320040
-
The usefulness of ‘in vivo’ antigens in the diagnosis of human Lyme borreliosis.
Zajkowska, J., Kondrusik, M., Grygorczuk, S., Pancewicz, S., Iżycka, A., 2008. International Journal of Medical Microbiology, Proceedings IX. International Jena Symposium on Tick-borne Diseases March 15-17, 2007 298, 361–364.
-
M, K., S, G., B, S., B, W., A, R., S, P., J, Z., R, S., T, H.-S., 2007. Annals of Agricultural and Environmental Medicine 14
-
Pugliese, A., Beltramo, T., Torre, D., 2007. Cell Biochemistry and Function 25, 185–188.
-
Zajkowska, J.M., Kondrusik, M., Pancewicz, S.A., Grygorczuk, S., Jamiołkowski, J., Stalewska, J., 2007. Pol Merkur Lekarski 23, 95–99
PMID:18044336
-
Chmielewska-Badora, J., Cisak, E., Wójcik-Fatla, A., Zwoliński, J., Buczek, A., Dutkiewicz, J., 2006. Ann Agric Environ Med. 13, 307–311
-
Pancewicz, S.A., Skrzydlewska, E., Hermanowska-Szpakowicz, T., Makieła, M., Kondrusik, M., Zajkowska, J., Grygorczuk, S., Swierzbińska, R., 2006. Przegl Epidemiol 60 Suppl 1, 102–108
PMID:16909786
-
Pietruczuk, A., Świerzbińska, R., Pancewicz, S., Pietruczuk, M., Hermanowska-Szpakowicz, T., 2006. Infection 34, 158–162.
-
Western-blot with VLSE protein and “in vivo” antigens in Lyme borreliosis diagnosis.
Zajkowska, J., Kondrusik, M., Pancewicz, S., Grygorczuk, S., Swierzbińska, R., Hermanowska-Szpakowicz, T., Czeczuga, A., Sienkiewicz, I., 2006. Przegl Epidemiol 60 Suppl 1, 177–185
PMID:16909799
-
Pancewicz, S.A., Skrzydlewska, E., Hermanowska-Szpakowicz, T., Stankiewicz, A., Sniecińska, A., Kondrusik, M., Zajkowska, J., Swierzbińska, R., 2005. Przegl Epidemiol 59, 35–41
PMID:16013408
-
Walory, J., Bukowska, B., Grzesiowski, P., Czarnecka, I., Paluchowska, E., Zabielski, S., Grzywocz, A., 2005. Pol Merkur Lekarski 19, 754–757
PMID:16521416
-
Zajkowska, J.M., Izycka, A., Jabłońska, E., Hermanowska-Szpakowicz, T., Kondrusik, M., Pancewicz, S., Grygorczuk, S., Swierzbińska, R., 2005. Pol Merkur Lekarski 19, 152–157
PMID:16245421
-
Židovec Lepej, S., Đaković Rode, O., Jeren, T., Vince, A., Remenar, A., Baršić, B., 2005. Journal of Neuroimmunology 163, 128–134.
-
Grygorczuk, S., Pancewicz, S., Zajkowska, J., Kondrusik, M., Świerzbińska, R., Hermanowska-Szpakowicz, T., 2004. Infection 32, 350–355.
-
Should ticks be regarded as a tularemia vector in habitants of North-Eastern Poland?
Pancewicz, S.A., Zajkowska, J.M., Swierzbińska, R., Kondrusik, M., Grygorczuk, S.S., Hermanowska-Szpakowicz, T., 2004. Med Pr 55, 189–192
PMID:15524088
-
Antinuclear antibodies are not increased in the early phase of Borrelia infection.
Spiewak, R.W., Stojek, N.M., Chmielewska-Badora, J., 2004. 11
-
Seroepidemiologic study on Lyme borreliosis in the Lublin region.
Chmielewska-Badora, J., 2003. Ann Agric Environ Med. 5, 183–186
-
Epidemiologic aspect of lyme borreliosis among the inhabitants of Podlasie Province.
Pancewicz, S.A., Olszewska, B., Hermanowska-Szpakowicz, T., Kondrusik, M., Zajkowska, J.M., Grygorczuk, S., Swierzbińska, R., 2001. Przegl Epidemiol 55 Suppl 3, 187–194
PMID:11984950
-
Enzyme immunoassay in the diagnosis of Lyme borreliosis.
Flisiak, R., Kalinowska, A., Bobrowska, E., Prokopowicz, D., 1996. Rocz Akad Med Bialymst 41, 83–89
PMID:8673810
-
Kondrusik, M., Daniluk, J., Hermanowska-Szpakowicz, T., 1995. Przegl Epidemiol 49, 257–260
PMID:7491420
 Download biomedica product list
Download biomedica product list